β-丙氨酸苄酯对甲苯磺酸盐 | 27019-47-2
中文名称
β-丙氨酸苄酯对甲苯磺酸盐
中文别名
3-氨基丙酸苄酯对甲苯磺酸盐;对甲苯磺酸beta-丙氨酸苄酯;beta-丙氨酸苄酯对甲苯磺酸盐;Β-氨基丙酸苄酯对甲苯磺酸盐;beta-Ala-OBzl稵osOH;b-氨基丙酸苄酯对甲苯磺酸盐;β-氨基丙酸苄酯对甲苯磺酸盐;Beta-丙氨酸苄酯对甲苯磺酸盐
英文名称
β-alanine benzyl ester p-toluenesulfonate
英文别名
β-alanine benzyl ester p-toluenesulfonate salt;3-(benzyloxy)-3-oxopropan-1-aminium 4-methylbenzenesulfonate;β-alaninebenzyl ester tosylate salt;β-alanine benzyl ester p-toluenesulphonate;beta-Alanine Benzyl Ester p-Toluenesulfonate;benzyl 3-aminopropanoate;4-methylbenzenesulfonic acid
CAS
27019-47-2
化学式
C7H7O3S*C10H14NO2
mdl
MFCD00039061
分子量
351.423
InChiKey
FRHWYVGCFUQMJR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:138-139°C
-
稳定性/保质期:
如果按照规格正确使用和储存,则不会分解。避免接触氧化物。
计算性质
-
辛醇/水分配系数(LogP):0.96
-
重原子数:24
-
可旋转键数:6
-
环数:2.0
-
sp3杂化的碳原子比例:0.235
-
拓扑面积:115
-
氢给体数:2
-
氢受体数:6
安全信息
-
WGK Germany:3
-
海关编码:2922499990
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:| -20℃ |
SDS
β-Alanine Benzyl Ester p-Toluenesulfonate Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: β-Alanine Benzyl Ester p-Toluenesulfonate
1
Revision number:
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols None
Signal word No signal word
Hazard statement None
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
β-Alanine Benzyl Ester p-Toluenesulfonate
Component(s):
Percent: >98.0%(T)
CAS Number: 27019-47-2
Chemical Formula: C10H13NO2·C7H8O3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
β-Alanine Benzyl Ester p-Toluenesulfonate
Section 5. FIRE-FIGHTING MEASURES
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
crystal - powder
Form:
Color: Very pale yellow - Pale yellow
No data available
Odor:
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
No data available
Flash Point:
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
β-Alanine Benzyl Ester p-Toluenesulfonate
Section 11. TOXICOLOGICAL INFORMATION
No data available
Skin corrosion/irritation:
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: β-Alanine Benzyl Ester p-Toluenesulfonate
1
Revision number:
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS Not classified
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols None
Signal word No signal word
Hazard statement None
Precautionary statements None
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
β-Alanine Benzyl Ester p-Toluenesulfonate
Component(s):
Percent: >98.0%(T)
CAS Number: 27019-47-2
Chemical Formula: C10H13NO2·C7H8O3S
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Rinse skin with
water/shower. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Dry chemical, foam, water spray, carbon dioxide.
Suitable extinguishing
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
β-Alanine Benzyl Ester p-Toluenesulfonate
Section 5. FIRE-FIGHTING MEASURES
When extinguishing fire, be sure to wear personal protective equipment.
Special protective
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Avoid contact with skin, eyes and clothing.
Advice on safe handling:
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
crystal - powder
Form:
Color: Very pale yellow - Pale yellow
No data available
Odor:
pH: No data available
Melting point/freezing point:No data available
Boiling Point/Range: No data available
No data available
Flash Point:
Explosive limits
No data available
Lower:
Upper: No data available
No data available
Density:
Solubility: No data available
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Nitrogen oxides (NOx), Sulphur oxides
Products:
Section 11. TOXICOLOGICAL INFORMATION
No data available
Acute Toxicity:
β-Alanine Benzyl Ester p-Toluenesulfonate
Section 11. TOXICOLOGICAL INFORMATION
No data available
Skin corrosion/irritation:
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
No data available
log Pow:
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Does not correspond to the classification standard of the United Nations
Hazards Class:
UN-No: Not Listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:描述:β-丙氨酸苄酯对甲苯磺酸盐 在 N-甲基吗啉 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 35.0h, 生成 L-4,4'-biphenylalanyl-β-alanine benzyl ester formate参考文献:名称:Structure-based design of dipeptide derivatives for the human neutral endopeptidase摘要:Neutral endopeptidase (NEP) plays a key role in the metabolic inactivation of various bioactive peptides such as atrial natriuretic peptide (ANP), endothelins, and enkephalins. Furthermore, NEP is known to work as elastase in skin fibroblast. Therefore, effective inhibitors of NEP offer significant therapeutic interest as antihypertensives, analgesics, and skin anti-aging agents. Recently, the X-ray crystal structure of human NEP complexed with phosphoramidon has been reported and provided insights into the active site specificity of NEP. Here, we designed new inhibitors by using in silico molecular modeling and synthesized them by short steps. Expectedly, we found highly effective inhibitors with sub-nanomolar levels of IC(50) values. These results indicate that our structure-based molecular designing program is useful for obtaining novel NEP inhibitors. Furthermore, these inhibitors may be attractive leads for the generation of new pharmaceuticals for NEP-related diseases. (C) 2011 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2011.08.064
-
作为产物:参考文献:名称:开发杂三芳基化合物作为亚型选择性和有效抑制 Sirt5 的新化学型摘要:与其他去乙酰化酶(NAD +依赖性 III 类赖氨酸脱酰基酶)相比,Sirt5 的抑制作用尚未得到很好的研究。我们目前的工作基于最近发现的 Sirt5 抑制剂巴柳氮,这是一种经批准的药物,口服后生物利用度可忽略不计。在之前的工作中首次了解其构效关系后,我们现在能够开发出杂芳基-三芳基化合物作为一种新型化学类型的药物样、强效和亚型选择性 Sirt5 抑制剂。铅结构的不利偶氮基团以系统和全面的方式进行了修饰,使我们得到了一些开链,最重要的是,五元杂环取代物(异恶唑CG_209、三唑CG_220、吡唑CG_232) 具有非常令人鼓舞的体外活性(Sirt5 上的 IC 50在低微摩尔范围内,<10 μM)。这些先进的抑制剂没有细胞毒性,并显示出良好的药代动力学特性,这通过活细胞成像实验对线粒体的渗透性得到了证实。此外,与作为参考化合物的巴柳氮相比,计算类似物的相对游离结合亲和力的结果与体外获得的抑DOI:10.1016/j.ejmech.2022.114594
-
作为试剂:描述:N-苄氧羰基-L-天门冬氨酸 4-叔丁酯 、 H-Trp-Pro-OTmse 在 palladium on activated charcoal N-甲基吗啉 、 1-羟基苯并三唑 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 β-丙氨酸苄酯对甲苯磺酸盐 、 氢气 作用下, 以 乙酸乙酯 、 水 为溶剂, 反应 0.5h, 生成 H-Asp(OBut)-Trp-Pro-OTmse参考文献:名称:Application of substituted 2-(trimethylsilyl)ethyl esters to suppress diketopiperazine formation摘要:The use of differently substituted 2-(trimethylsilyl)ethyl esters for C-terminal protection in peptide synthesis has been investigated. While the use of the unsubstituted 2-(trimethylsilyl)ethyl ester resulted in a substantial amount of diketopiperazine at the dipeptide stage, use of the corresponding methyl-substituted silyl ester gave a significant reduction of this undesired pathway. Both esters could be deprotected by fluoride-induced cleavage under mild conditions. (C) 2004 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2004.03.054
文献信息
-
A conjugate from a laminin-related peptide, Tyr-Ile-Gly-Ser-Arg, and chitosan: efficient and regioselective conjugation and significant inhibitory activity against experimental cancer metastasis作者:Yasuhiro Nishiyama、Tomoko Yoshikawa、Nobumichi Ohara、Keisuke Kurita、Keiko Hojo、Haruhiko Kamada、Yasuo Tsutsumi、Tadanori Mayumi、Koichi KawasakiDOI:10.1039/a908145c日期:——A laminin-related antimetastatic peptide was conjugated with chitosan, and antimetastatic activity of the peptide–chitosan conjugate was assayed. Chitosan was converted to its organosoluble derivative, 6-O-trityl-chitosan, in 3 steps, and then coupled with the peptide portion, Ac-Tyr-Ile-Gly-Ser-Arg-βAla-OH (βAla; β-alanine), which contains a spacer amino acid at the carboxy-terminus. The product was treated with CHCl2CO2H to afford the desired conjugate, Ac-Tyr-Ile-Gly-Ser-Arg-βAla–chitosan. The peptide was introduced to every 6.3 glucosamine residues. The conjugate proved to have higher inhibitory activity against experimental lung metastasis of B16BL6 melanoma cells in mice than did the parent peptide.
-
<i>N</i>-Acylanilines, Herbicide−CHA Chimera, and Amino Acid Analogues as Novel Chemical Hybridizing Agents for Wheat (<i>Triticum aestivum</i> L.)作者:Kajal Chakraborty、C. DevakumarDOI:10.1021/jf051458t日期:2005.10.1development, chemical induction of male sterility mediated technology based on chemical hybridizing agents (CHAs) holds a great potential. N-Acylaniline derivatives, namely, ethyl 4'-fluoro oxanilate (1) and ethyl 4'-trifluoromethyl oxanilate (2) containing halogen atoms in the para position of the aryl ring and substituted amide linkage (-CO-NH-) in the acyl side chain induced >98% spikelet sterility on three在没有可行的杂交小麦开发替代技术的情况下,基于化学杂交剂(CHA)的雄性不育介导技术的化学诱导具有巨大的潜力。N-酰基苯胺衍生物,即在芳基环对位含有卤素原子并在其芳基中的取代酰胺键(-CO-NH-)的4'-氟代草酸乙酯(1)和4'-三氟代甲基草酸乙酯(2)。酰基侧链在1500 ppm时对三种基因型小麦,即PBW 343,HD 2046和HD 2733诱导的小穗不育性> 98%。以甘氨酸和丙氨酸为模板,将活性部分以除草剂-CHA嵌合体和氨基酸类似物的形式掺入。通过合成除草剂的嵌合结构,可以使目标活性更具选择性(2,4-二氯苯氧基乙酸和dalapon)和最活跃的CHA模板,即4-氟苯胺基和β-乙氧羰基部分。在除草剂-CHA嵌合体中,2',4'-草酸二氯苯基乙酯(14)诱导了79.11%的雄性不育,而2-氧代-3-氮杂氮杂双苄基甲基酯(20)最佳,在HD 2733中诱导了73.87%的雄性不育。
-
Cell‐Based Optimization of Covalent Reversible Ketoamide Inhibitors Bridging the Unprimed to the Primed Site of the Proteasome β5 Subunit作者:Daniel Stubba、Dennis Bensinger、Janika Steinbacher、Lilia Proskurjakov、Álvaro Salcedo Gómez、Uwe Schmidt、Stefan Roth、Katja Schmitz、Boris SchmidtDOI:10.1002/cmdc.201900472日期:2019.12.4boronates, optimization of novel covalent-reversibly binding warheads remains largely unattended. We previously reported α-ketoamides to be a promising reversible lead motif, yet the cytotoxic activity required further optimization. This work focuses on the lead optimization of phenoxy-substituted α-ketoamides combining the structure-activity relationships from the primed and the non-primed site of the泛素-蛋白酶体系统(UPS)是已批准的治疗特定血液系统恶性肿瘤药物的既定治疗靶标。虽然针对UPS的药物发现侧重于不可逆结合的环氧酮和缓慢可逆结合的硼酸酯,但新型共价可逆结合弹头的优化在很大程度上无人值守。我们之前曾报道过α-酮酰胺是一种有前途的可逆铅基序,但其细胞毒性活性需要进一步优化。这项工作着眼于苯氧基取代的α-酮酰胺的前导优化,结合了蛋白酶体β5亚基的引发和未引发位点之间的结构-活性关系。我们的优化策略伴随着分子建模,表明P1'被占领 通过3-苯氧基团增加白血病细胞系中β5的抑制作用和细胞毒活性。进一步分析关键化合物的时间依赖性抑制细胞底物转化。此外,与硼替佐米相反,α-酮酰胺前导结构27不会影响Danio rerio胚胎的逃避反应行为,这表明靶标特异性增加。
-
NOVEL BINDER-DRUG CONJUGATES (ADCs) AND USE OF SAME申请人:SEATTLE GENETICS, INC.公开号:US20150246136A1公开(公告)日:2015-09-03The present patent application relates to novel binder-drug conjugates (ADCs) of N,N-dialkylauristatins directed against the target epidermal growth factor receptor (EGFR, gene ID 1956), effective metabolites of these ADCs, methods for producing these ADCs, use of these ADCs for treatment and or prevention of diseases as well as the use of these ADCs to produce pharmaceutical drugs for treatment and/or prevention of diseases, in particular hyperproliferative and/or angiogenic diseases such as cancer, for example. Such treatments may be administered as monotherapy or in combination with other pharmaceutical drugs or other therapeutic measures.
-
Synthesis of Tn, Sialyl Tn and HIV-1-Derived Peptide Antigen Conjugates Having a Lipid A Analog as an Immunoadjuvant for Synthetic Vaccines.作者:Keisuke MIYAJIMA、Takahiro NEKADO、Kiyoshi IKEDA、Kazuo ACHIWADOI:10.1248/cpb.46.1676日期:——Conjugates (3-5) of Tn, sialyl Tn and HIV-1-derived peptide antigen with a N-tetradecanoyl L-serine-β-alanine-containing D-glucosamine derivative, structurally related to lipid A, as an immunoadjuvant for the development of totally synthetic vaccines against cancers or HIV were synthesized. The mitogenic activity of compounds 3, 4 and 5 was stronger than that of lipid A analogs (1, 2).
表征谱图
-
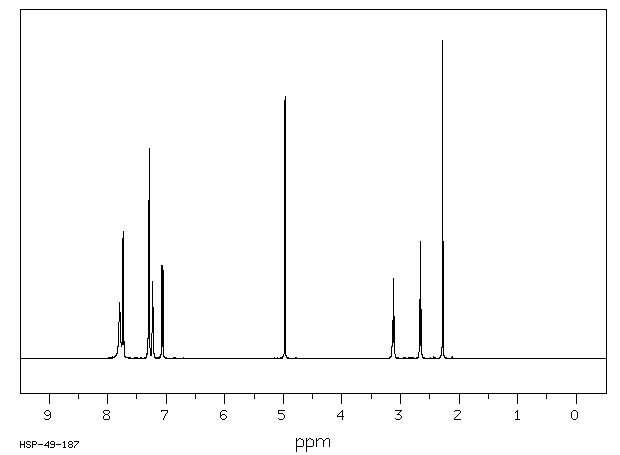
氢谱1HNMR
-
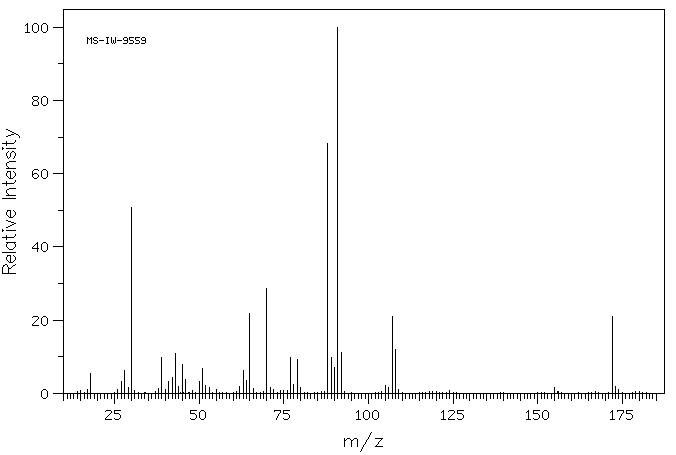
质谱MS
-
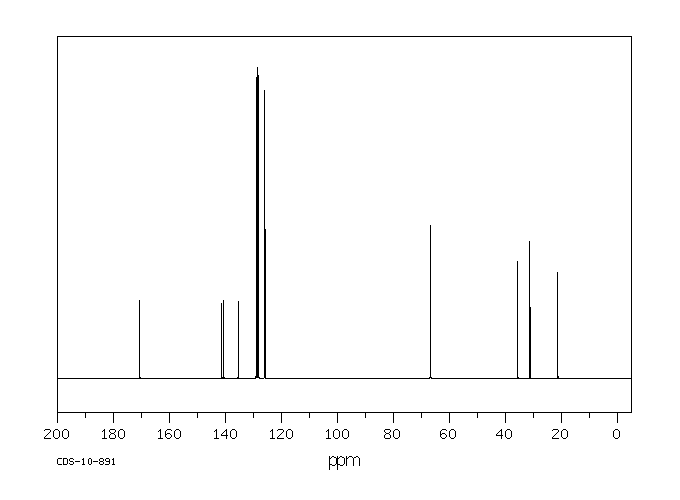
碳谱13CNMR
-
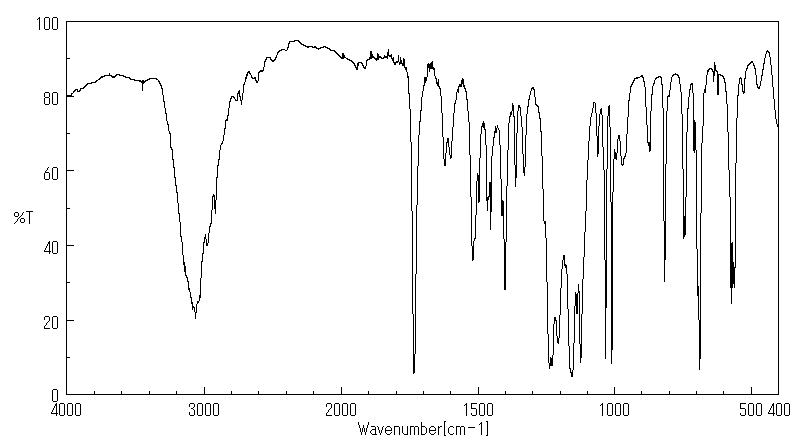
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸