乐果(有机磷杀虫、杀螨剂)-D6 | 35946-91-9
中文名称
乐果(有机磷杀虫、杀螨剂)-D6
中文别名
2,5-二异丙基苯酚;2,4-二羟基-8-甲氧基喹啉
英文名称
2,5-Diisopropylphenol
英文别名
2,5-di(propan-2-yl)phenol
CAS
35946-91-9
化学式
C12H18O
mdl
——
分子量
178.274
InChiKey
VFNUNYPYULIJSN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:118-119 °C
-
沸点:115-120 °C
-
密度:0.948±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
-
物理描述:Solid
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:13
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2907199090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:2-8℃,惰性气体
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3-异丙基苯酚 3-isopropylhydroxybenzene 618-45-1 C9H12O 136.194 —— 1-(2-hydroxy-4-isopropylphenyl)ethanone 91969-72-1 C11H14O2 178.231 2,4-二异丙基苯酚 2,4-diisopropylphenol 2934-05-6 C12H18O 178.274 3,5-双(1-甲基乙基)苯酚 3,5-diisopropylphenol 26886-05-5 C12H18O 178.274 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,4-二异丙基苯酚 2,4-diisopropylphenol 2934-05-6 C12H18O 178.274 3,5-双(1-甲基乙基)苯酚 3,5-diisopropylphenol 26886-05-5 C12H18O 178.274
反应信息
-
作为反应物:描述:乐果(有机磷杀虫、杀螨剂)-D6 在 lithium aluminium tetrahydride 、 sodium hydride 作用下, 以 乙醚 为溶剂, 反应 22.0h, 生成 2,5-diisopropylthiophenol参考文献:名称:4-羟基-5,6-二氢吡喃酮。2.有效的HIV蛋白酶的非肽抑制剂。摘要:4-羟基-5,6-二氢吡喃酮模板被用作柔性支架,从该支架可构建有效的HIV蛋白酶活性位点抑制剂。使用类似的结合模式在HIV蛋白酶的活性位点中模拟了二氢吡喃酮1c(5,6-二氢-4-羟基-6-苯基-3-[(2-苯基乙基)硫代] -2H-吡喃-2-酮)发现为先前报道的4-羟基苯并吡喃-2-酮。我们的模型导致我们追求6,6-二取代的二氢吡喃酮的合成,目的是填充S1和S2,从而提高未填充S2的母体二氢吡喃酮1c的效力。为此,我们在二氢吡喃酮的6-位连接了各种疏水和亲水侧链,以模拟天然和非天然氨基酸,已知它们是P2和P2'的有效底物。将母体二氢吡喃酮1c(IC50 = 2100 nM)制成化合物,其效力提高了100倍以上[18c,IC50 = 5 nM,5-(3,6-dihydro-4-hydroxy-6-oxo-2-苯基-5- [2-苯基乙基)硫代] -2H-吡喃-2-基)戊酸和12c,IC50 =DOI:10.1021/jm970615f
-
作为产物:参考文献:名称:2,5-DIALKYL-4-H/HALO/ETHER-PHENOL COMPOUNDS摘要:本公开提供用于治疗诸如惊厥和震颤等神经学状况的酚类化合物,其具有公式(I)的结构: 其中R2、R4和R5如详细描述中定义;包含至少一种该化合物的药物组合物;以及用于治疗神经学状况的方法。公开号:US20150148430A1
-
作为试剂:描述:参考文献:名称:未保护的碳水化合物的选择性催化氧化摘要:对未保护的碳水化合物进行直接催化功能化的新策略的开发将是糖科学的一个有利的进展。在本文中,我们报道了未保护的碳水化合物的催化氧化可以选择性地与[[neocuproine)Pd(OAc)] 2(OTf)2(1)进行,以生成3-酮糖。在酚类添加剂存在下,Pd含量低至1%即可进行催化好氧氧化。使用Pd催化剂1催化乙腈或乙腈/水中的各种未保护吡喃糖苷的催化氧化1用氧气或苯醌选择性地生成3-酮糖。少量的4-酮糖竞争性地形成,特别是在吡喃糖苷在吡喃糖苷的C4处带有轴向取代基的情况下。催化氧化也可以在三氟乙醇中进行,但是对于在C2或C4处带有轴向取代基的吡喃糖苷,选择性氧化成3-酮糖伴随着差向异构化作用,得到了赤道的3-酮糖。未保护的六呋喃糖苷或唾液酸衍生物的催化氧化选择性地在三氟乙醇中的环外二醇或三醇处发生,以生成环外羟基酮。DOI:10.1021/acscatal.6b01501
文献信息
-
PROCESS FOR THE PREPARATION OF AROMATIC AMINES申请人:AHRENS SEBASTIAN公开号:US20120071692A1公开(公告)日:2012-03-22A process for preparing an aromatic amine by reacting a corresponding aromatic alcohol with an aminating agent selected from the group consisting of ammonia, primary amines and secondary amines, in the presence of hydrogen and a catalyst molding, at a temperature of from 60-300°. The catalyst molding comprises Zr, Pd and Pt and has an annular tablet form with an external diameter in the range from 2-6 mm, a height in the range from 1-4 mm and an internal diameter of from 1-5 mm or a topologically equivalent form with the same volume. Catalyst moldings comprising Zr, Pd and Pt are also provided. The catalyst molding has an annular tablet form with an external diameter in the range from 3-6 mm, a height in the range from 1-4 mm and an internal diameter of from 2-5 mm or a topologically equivalent form.
-
[EN] 2,5-DIALKYL-4-H/HALO/ETHER-PHENOL COMPOUNDS<br/>[FR] COMPOSÉS 2,5-DIALKYL-4-H/HALO/ÉTHER-PHÉNOL申请人:TANSNA THERAPEUTICS INC公开号:WO2016190871A1公开(公告)日:2016-12-01The present disclosure provides phenolic compounds useful in the treatment of neurological conditions such as convulsions and tremors, having the structure of Formula (I): wherein R2, R4, R5, & R6, are as defined in the detailed description; pharmaceutical compositions comprising at least one of the compounds; and methods for treating neurological conditions.本公开提供了对神经系统疾病如抽搐和震颤有用的酚类化合物,其结构如下式(I):其中R2、R4、R5和R6如详细描述中所定义;包括至少一种该化合物的药物组合物;以及治疗神经系统疾病的方法。
-
OPTICAL RECORDING MEDIUM AND COMPOUND USED FOR THE SAME申请人:SHIOZAKI Hiroyoshi公开号:US20090306376A1公开(公告)日:2009-12-10A compound comprising a ring structure including a ring composed of four carbon atoms and two nitrogen atoms and a substituted or unsubstituted amino group bonded to the ring structure.一种化合物,包括一个环结构,其中包括由四个碳原子和两个氮原子组成的环,以及与环结构相结合的取代或未取代的氨基团。
-
Oral care compositions with color changing indicator申请人:Sabnis W. Ram公开号:US20060222601A1公开(公告)日:2006-10-05The invention describes color changing toothpastes and mouthwashes which contains acid-base indicator(s) for interaction with the oral cavity to provide a color change indicative of treatment time.
-
The Reaction of Nitrile Oxide-Quinone Cycloadducts. III. Reinvestigation of the Base-Induced Isomerization of the 1 : 1 -C=C-Adducts of Aromatic Nitrile Oxides with 2,5- and 2,6-Dialkyl-Substituted<i>p</i>-Benzoquinones作者:Takashi Mukawa、Yukihiko Inoue、Shinsaku ShiraishiDOI:10.1246/bcsj.72.2549日期:1999.11The structure of the product obtained by the base-induced isomerization of the 1,3-dipolar cycloadduct of 2,5-di-t-butyl-p-benzoquinone with 2,6-dichlorobenzonitrile oxide was determined by X-ray analysis. The t-butyl group at the bridgehead position of the 1,3-dipolar cycloadduct migrated to the neighboring carbonyl carbon atom. This base-induced rearrangement took place with a bulky group, i. e., Et, i-Pr, t-Bu, and Bn at the bridgehead position of nitrile oxide-quinone cycloadducts in an alcoholic media. The driving force of this reaction is considered to be due to stabilization by aromatization from isoxazoline derivatives to isoxazole-fused p-quinol derivatives.
表征谱图
-
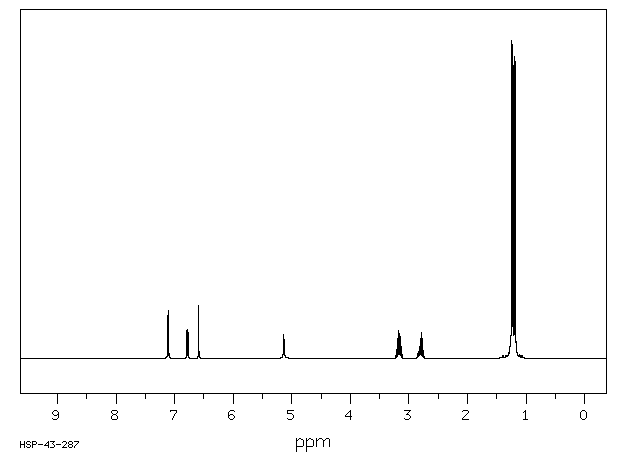
氢谱1HNMR
-
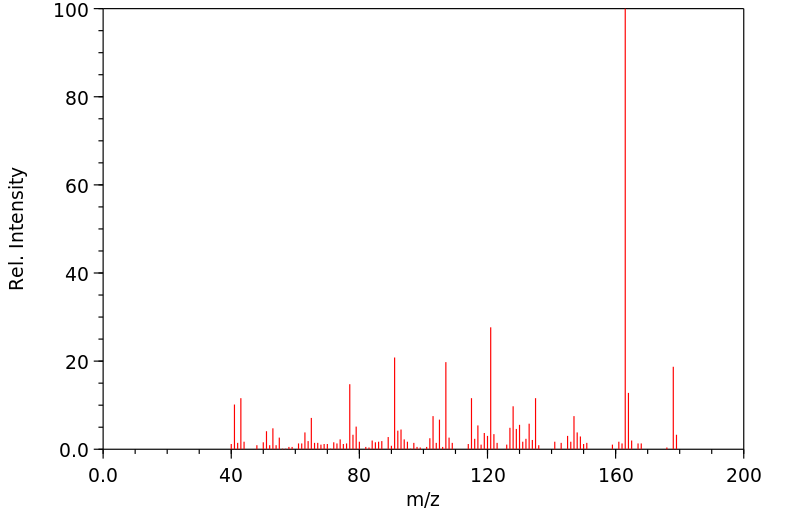
质谱MS
-
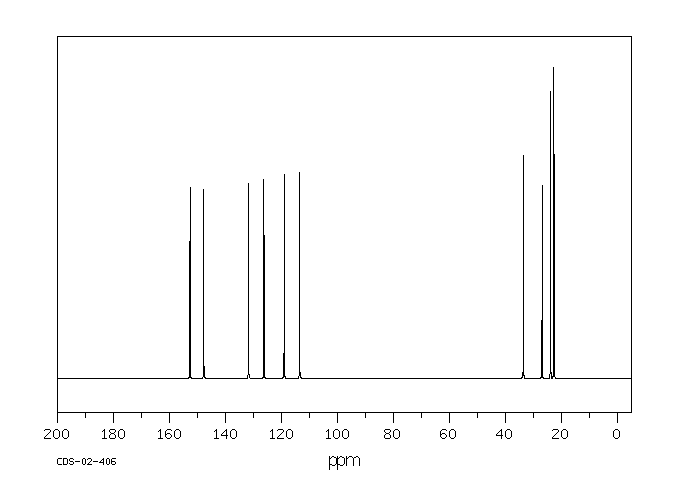
碳谱13CNMR
-
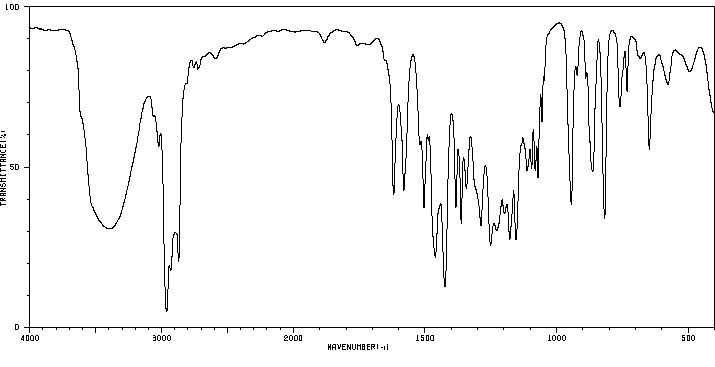
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸