乙二醇乙醚 | 110-80-5
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-100 °C
-
沸点:135 °C(lit.)
-
密度:0.93 g/mL at 25 °C(lit.)
-
蒸气密度:3.1 (vs air)
-
闪点:107.6 °F
-
溶解度:水:混溶
-
最大波长(λmax):λ: 215 nm Amax: 1.00λ: 225 nm Amax: 0.50λ: 250 nm Amax: 0.20λ: 305 nm Amax: 0.01
-
暴露限值:TLV-TWA skin 5 ppm (18.5 mg/m3) (ACGIH). .
-
介电常数:29.6(24℃)
-
LogP:0.32 at 20℃
-
物理描述:Ethylene glycol monoethyl ether appears as a clear colorless liquid. Flash point of 120°F. Less dense than water. Its vapors are heavier than air.
-
颜色/状态:Colorless liquid
-
气味:Sweet, pleasant, ether-like odor
-
味道:SLIGHTLY BITTER
-
蒸汽密度:3.1 (NTP, 1992) (Relative to Air)
-
蒸汽压力:5.31 mm Hg at 25 °C
-
亨利常数:4.70e-07 atm-m3/mole
-
大气OH速率常数:1.54e-11 cm3/molecule*sec
-
稳定性/保质期:
-
稳定性:稳定。
-
禁配物:强氧化剂、酸类、碱类。
-
避免接触的条件:空气、光照。
-
聚合危害:不聚合。
-
-
自燃温度:455 °F (235 °C)
-
分解:Hazardous decomposition products formed under fire conditions. - Carbon oxides
-
粘度:1.84 centipoise at 25 °C
-
汽化热:48.21 kJ/mol at 25 °C
-
表面张力:28.2 dynes/cm at 25 °C
-
气味阈值:...Odor threshold of about 25 ppm and a strong odor at about 50 ppm.
-
折光率:Index of refraction: 1.4054 at 25 °C/D
-
解离常数:pKa = 14.8
-
保留指数:702;702;687;694;693.1;691.7;695;695;694;694;701;701
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:6
-
可旋转键数:3
-
环数:0.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:29.5
-
氢给体数:1
-
氢受体数:2
ADMET
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 0.5 ppm (1.8 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:500 ppm
-
危险品标志:T
-
安全说明:S45,S53
-
危险类别码:R60,R20/21/22,R10,R61
-
WGK Germany:1
-
海关编码:2909499000
-
危险品运输编号:UN 1171 3/PG 3
-
危险类别:3
-
RTECS号:KK8050000
-
包装等级:III
-
危险标志:GHS02,GHS06,GHS08
-
危险性描述:H226,H302,H331,H360
-
危险性防范说明:P201,P210,P280,P304 + P340 + P312,P308 + P313,P370 + P378
-
储存条件:储存注意事项: - 储存于阴凉、通风的库房。 - 远离火种、热源,库温不宜超过37℃。 - 包装要求密封,不可与空气接触。 - 应与氧化剂、酸类、碱类分开存放,切忌混储。 - 采用防爆型照明、通风设施。 - 禁止使用易产生火花的机械设备和工具。 - 储区应备有泄漏应急处理设备和合适的收容材料。
制备方法与用途
乙二醇乙醚是一种乙二醇单醚类化合物,结构中含有醚键、羟基和不同的烷基。它既具有水溶性,又能溶解有机物分子、合成聚合物及天然高分子,是一种通用性的绿色溶剂,在工业上广泛应用作溶剂,同时也可用作防冻液、表面活性剂、印刷线路板粘结剂、护肤品添加剂等,展现出广阔的应用前景。
制备方法将46.0g Mg(NO₃)₂·6H₂O 和13.1g Co(NO₃)₂·6H₂O溶解于100ml蒸馏水中,再加入1.4g La(NO₃)₃·6H₂O,得到盐溶液。随后将该溶液与25%的浓氨水按并流滴加的方式混合,并调节混合溶液pH值为9,在常压和40℃下反应1小时。冷却至室温后老化20小时,再通过常规抽滤、洗涤、烘干步骤,并在500℃焙烧4小时,得到块状催化剂,粉碎至40-60目,最终制得所需的催化剂标记为GM1。
将所制备的催化剂GM1置于连续膜反应器中的管式膜内,外部用氮气进行吹扫。在常压条件下,摩尔比为3∶1的乙醇与环氧乙烷进行气相乙氧基化反应,其中乙醇先在90℃预热器中气化,并且膜反应器温度保持在100℃,气体空速为1.5×10³ml·g⁻¹·h⁻¹。通过该步骤可以制得乙二醇乙醚。
化学性质乙二醇乙醚是一种无色液体,几乎无味,并且可与水、乙醇、乙醚、丙酮及液体酯类混溶。此外,它还能溶解多种油类、树脂和蜡等物质。
用途- 用作溶剂、萃取剂、防水剂以及有机中间体。
- 作为硝基赛璐珞、假漆的溶剂,皮革着色剂及乳化液稳定剂。
- 是低挥发性溶剂。适用于硝基纤维素漆和飞机翼涂料的溶剂,还可用作清漆的涂膜剂,净化液、染料浴、水溶性颜料与染料溶液,精炼皮革的溶剂,并能增加乳胶的稳定性。
乙二醇乙醚可通过环氧乙烷与乙醇反应制得。在25-30℃下,缓慢将环氧乙烷加入无水乙醇中,待温度升至70℃后完成反应并放置过夜。回收乙醇后使用10%氢氧化钠溶液中和至pH=8,并进行分馏得到粗品;再经精馏收集133.5-135.5℃的馏分为成品。每吨产品消耗环氧乙烷863kg及无水乙醇1020kg。
分类- 易燃液体
- 毒性分级:中毒
- 口服大鼠LD₅₀: 2125毫克/公斤;小鼠口服LD₅₀: 2451毫克/公斤
- 刺激数据:
- 皮肤-兔子:500毫克,轻度刺激
- 眼睛-兔子:50毫克,中度刺激
- 爆炸物危险特性:蒸汽与空气混合可爆炸
- 可燃性危险特性:易燃;火场释放辛辣刺激烟雾
库房需保持通风、低温干燥,并与其他氧化剂分开存放和运输。
灭火剂 职业标准- TLV-TWA:19毫克/立方米
- STEL:40毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二乙二醇 diethylene glycol 111-46-6 C4H10O3 106.122 乙二醇二乙醚 monoethylene glycol diethyl ether 629-14-1 C6H14O2 118.176 二乙二醇单甲醚 2-(2-methoxyethoxy)ethyl alcohol 111-77-3 C5H12O3 120.148 2-氯乙氧基乙醇 2-(2-Chloroethoxy)ethanol 628-89-7 C4H9ClO2 124.567 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 二乙二醇 diethylene glycol 111-46-6 C4H10O3 106.122 1,4-二氧六环 1,4-dioxane 123-91-1 C4H8O2 88.1063 乙二醇甲酸乙醚 1-methoxy-2-ethoxyethane 5137-45-1 C5H12O2 104.149 乙二醇二乙醚 monoethylene glycol diethyl ether 629-14-1 C6H14O2 118.176 乙醚 diethyl ether 60-29-7 C4H10O 74.1228 二乙二醇乙醚 ethoxyethoxyethanol 111-90-0 C6H14O3 134.175 —— 3,6,9,12-tetraoxatetradecane 4499-99-4 C10H22O4 206.282 二乙二醇二乙醚 3,6,9-trioxaundecane 112-36-7 C8H18O3 162.229 2-乙氧基乙基乙烯醚 1-ethoxy-2-vinyloxy-ethane 18861-14-8 C6H12O2 116.16
反应信息
-
作为反应物:描述:参考文献:名称:GB377678摘要:公开号:
-
作为产物:描述:参考文献:名称:[EN] COMPOSITIONS, SYNTHESIS, AND METHODS OF USING PHENYLCYCLOALKYLMETHYLAMINE DERIVATIVES
[FR] COMPOSITIONS, SYNTHÈSES ET PROCÉDÉS D'UTILISATION DE DÉRIVÉS DE PHÉNYLCYCLOALKYLMÉTHYLAMINE摘要:公开号:WO2013102195A8 -
作为试剂:参考文献:名称:Brain Pathology: Past, Present, and Future摘要:DOI:10.1111/j.1750-3639.2000.tb00253.x
文献信息
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS申请人:Carroll William A.公开号:US20090105306A1公开(公告)日:2009-04-23The present invention relates compounds of formula (I) wherein A and R 1 are as defined in the specification, pharmaceutical compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and pharmaceutical compositions.本发明涉及以下式(I)的化合物 其中A和R1如规范中所定义,包括这些化合物的药物组合物,以及使用这些化合物和药物组合物治疗疾病和疾病的方法。
-
COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS申请人:Carroll William A.公开号:US20100249129A1公开(公告)日:2010-09-30Disclosed herein are compounds of formula (I) wherein Ring A and R 1 are as defined in the specification. Pharmaceutical compositions comprising such compounds, and methods for treating conditions and disorders using such compounds and pharmaceutical compositions are also disclosed.本文揭示了以下式(I)的化合物 其中环A和R 1 如规范中所定义。还披露了包含这些化合物的药物组合物,以及使用这些化合物和药物组合物治疗疾病和疾病的方法。
-
Synthesis, antiproliferative activity and molecular docking of thiocolchicine urethanes作者:Urszula Majcher、Alicja Urbaniak、Ewa Maj、Mahshad Moshari、Magdalena Delgado、Joanna Wietrzyk、Franz Bartl、Timothy C. Chambers、Jack A. Tuszynski、Adam HuczyńskiDOI:10.1016/j.bioorg.2018.09.004日期:2018.12colchicine exert their therapeutic effect by changing the dynamics of tubulin and its polymer form, microtubules. The identification of tubulin as a potential target for anticancer drugs has led to extensive research followed by clinical development of numerous compounds from several families. In this paper we report on the design, synthesis and in vitro evaluation of a group of thiocolchicine derivatives许多天然存在的化合物(例如紫杉醇,长春碱,康维他汀和秋水仙碱)通过改变微管蛋白及其聚合物形式(微管)的动力学来发挥其治疗作用。将微管蛋白鉴定为抗癌药物的潜在靶标导致了广泛的研究,随后进行了来自多个家族的许多化合物的临床开发。在本文中,我们报告了一组在环B处修饰的硫代秋水仙碱衍生物的设计,合成和体外评估,该化合物在此处标记为化合物4 – 14。这些化合物是通过7-脱乙酰基-10-硫代秋水仙碱3的简单反应获得的在三光气存在下与十一种不同的醇一起使用。这些新型药物已针对四种人类癌细胞系进行了抗增殖活性检查,其作用方式已通过分子对接证实为秋水仙碱结合位点抑制(CBSI)。分子模拟为测试化合物提供了合理的微管蛋白结合模型。上的基础的体外试验中,衍生物4 - 8和14表现出对MCF-7,的LoVo和A549肿瘤细胞系(IC最高效力50值= 0.009–0.014μM)。与包括顺铂和阿霉素以及未修饰秋水
-
Cobalt-catalyzed regioselective cross-dehydrogenative C O coupling of 1-naphthylamide derivatives with diols作者:Mengfan Zhang、Zhen Yang、Ruipeng Li、Xianqiang Huang、Ruokun Feng、Chenze QiDOI:10.1016/j.tetlet.2020.151592日期:2020.3The cobalt-catalyzed regioselective C-H alkoxylation of 1-naphthylamide with diols through a bidentate-chelation assistance has been developed. In this transformation, not only linear diols, but also branched diols and oligoethylene glycols were tolerated under current reaction conditions, affording the corresponding hydroxyalkyl aryl ethers. In addition, control experiments suggested that picolinoyl
-
[EN] PYRROLOPYRIMIDINES<br/>[FR] PYRROLOPYRIMIDINES申请人:JANSSEN PHARMACEUTICA NV公开号:WO2009016132A1公开(公告)日:2009-02-05The present invention relates to compounds or pharmaceutically-acceptable salts thereof, processes for preparing them, pharmaceutical compositions containing them and their use in therapy. The invention particularly relates to compounds that are polo-like kinase (PLKs) inhibitors useful for the treatment of disease states mediated by PLK, especially PLK4, in particular such compounds that are useful in the treatment of pathological processes which involve an aberrant cellular proliferation, such as tumour growth, rheumatoid arthritis, restenosis and atherosclerosis.本发明涉及化合物或其药用盐,制备它们的方法,含有它们的药物组合物以及它们在治疗中的用途。该发明特别涉及一类极化样激酶(PLKs)抑制剂化合物,用于治疗由PLK介导的疾病状态,特别是PLK4,特别是在治疗涉及异常细胞增殖的病理过程中有用的化合物,如肿瘤生长、类风湿性关节炎、再狭窄和动脉粥样硬化。
表征谱图
-
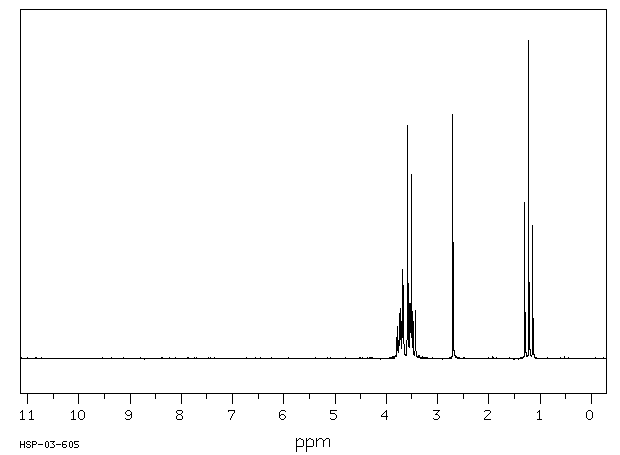
氢谱1HNMR
-
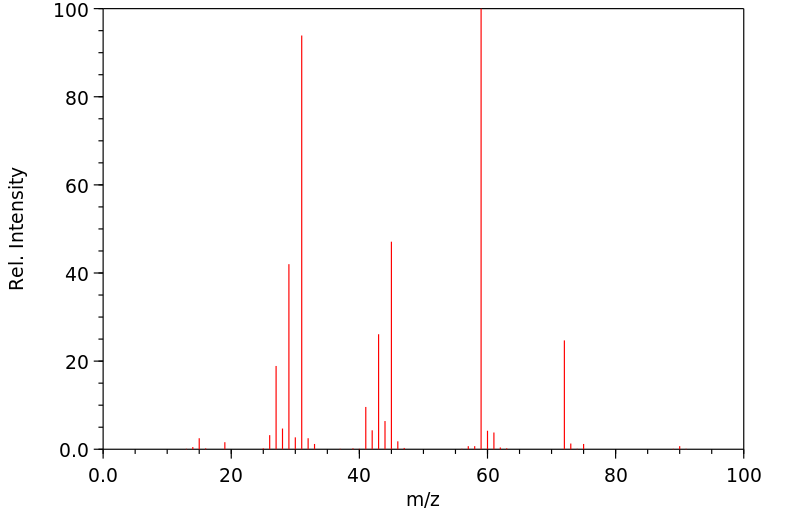
质谱MS
-
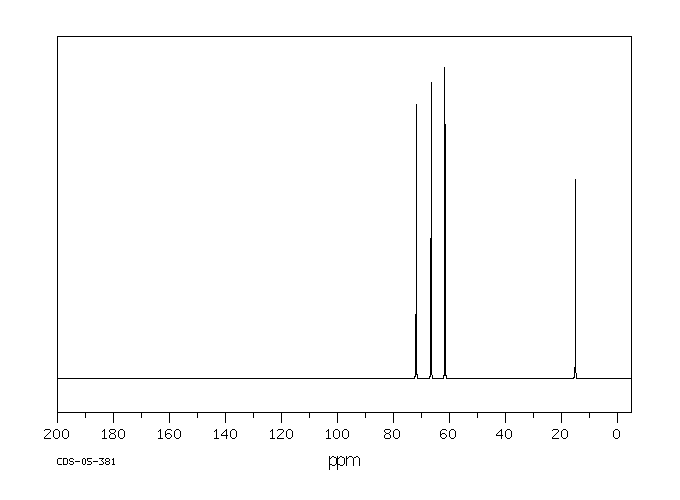
碳谱13CNMR
-
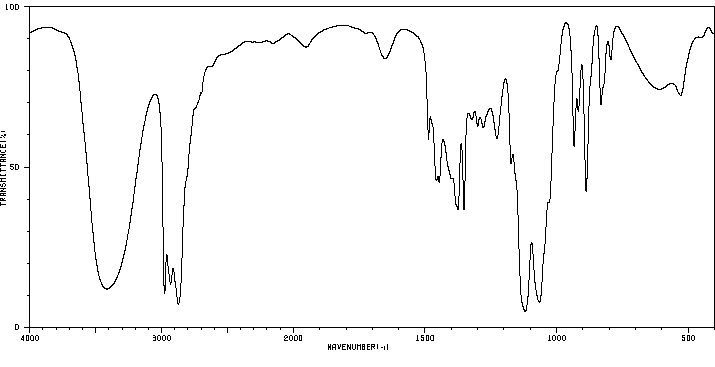
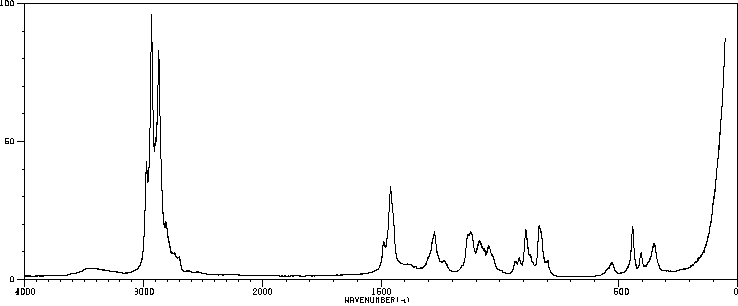
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息