乙二醇乙醚醋酸酯 | 111-15-9
中文名称
乙二醇乙醚醋酸酯
中文别名
乙二醇乙醚乙酸酯;1-乙酰氧基-2-乙醚;2-乙氧基乙基乙酸酯;2-乙氧基乙酸乙酯;乙酸-2-乙氧基乙酯;CAC;乙基溶纤剂醋酸酯
英文名称
2-Ethoxyethyl acetate
英文别名
——
CAS
111-15-9
化学式
C6H12O3
mdl
MFCD00009251
分子量
132.159
InChiKey
SVONRAPFKPVNKG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-61 °C
-
沸点:156 °C(lit.)
-
密度:0.975 g/mL at 25 °C(lit.)
-
蒸气密度:4.6 (vs air)
-
闪点:135 °F
-
溶解度:可溶于约6份的水中
-
介电常数:7.6(30℃)
-
暴露限值:NIOSH REL: TWA 0.5 ppm (2.7 mg/m3), IDLH 500 ppm; OSHA PEL: TWA 100 ppm (540 mg/m3); ACGIH TLV: TWA 5 ppm (adopted).
-
LogP:0.449 (est)
-
物理描述:Ethylene glycol monoethyl ether acetate appears as a clear colorless liquid with a pleasant odor. Flash point of 120°F. Less dense than water. Vapors are heavier than air.
-
颜色/状态:Colorless liquid
-
气味:MILD ESTER-LIKE ODOR BECOMES OBJECTIONABLE IN HIGH CONCN ...
-
味道:... A BITTER ACID TASTE
-
蒸汽密度:4.72 (NTP, 1992) (Relative to Air)
-
蒸汽压力:2.00 mm Hg at 25 °C
-
亨利常数:3.20e-06 atm-m3/mole
-
大气OH速率常数:1.30e-11 cm3/molecule*sec
-
自燃温度:715 °F (379 °C)
-
分解:WHEN HEATED TO DECOMPOSITION IT EMITS ACRID SMOKE AND IRRITATING FUMES.
-
粘度:1.32 cP at 20 °C
-
燃烧热:-10,700 Btu/lb= -6,000 cal/g= -250X10X5 J/kg (est)
-
汽化热:130 Btu/lb= 74 cal/g= 3.1X10X5 J/kg
-
表面张力:31.8 dynes/cm at 25 °C
-
折光率:Index of refraction: 1.4054 at 20 °C
-
相对蒸发率:0.20 (n-butyl acetate = 1)
-
保留指数:880.8;888;882;875;877.7;859;876;876;874;876;872
-
稳定性/保质期:
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:9
-
可旋转键数:5
-
环数:0.0
-
sp3杂化的碳原子比例:0.833
-
拓扑面积:35.5
-
氢给体数:0
-
氢受体数:3
ADMET
代谢
... Its metabolic product is ethylene glycol monoethyl ether to which its systemic effects may be related.
来源:Hazardous Substances Data Bank (HSDB)
代谢
研究了在接触乙酸乙二醇单乙基醚的男性志愿者中乙氧基乙酸尿排泄的情况,乙氧基乙酸是该化合物的代谢产物。五名年龄在21至30岁之间的志愿者在静息状态下分别接触了14、28和50毫克/立方米的环境,另外五名志愿者在静息状态下以及进行30瓦和60瓦运动时接触了28毫克/立方米的环境。每个人在每个浓度下暴露时间为4小时。实验之间的未接触期持续了2到3周。乙氧基乙酸最大排泄发生在停止接触后3到4小时;消除的半衰期为23.6小时。研究发现,在体力运动后,观察到第二次排泄高峰,大约在第一次高峰后3小时。在大约42小时后,大约有22%的吸收乙酸乙二醇单乙基醚剂量以乙氧基乙酸的形式在尿液中排出。
The urinary excretion of ethoxyacetic acid was studied in male volunteers exposed to ethylene glycol monoethyl ether acetate, of which ethoxyacetic-acid is a metabolite. Five of the volunteers, aged 21 to 30 years, were exposed to 14, 28, and 50 mg/cu m at rest, and five were exposed to 28 mg/cu m at rest and during exercise at 30 and 60 watts. Individuals were exposed to each concentration for periods of 4 hours. Unexposed periods between the experiments lasted 2 to 3 weeks. Maximal ethoxyacetic acid excretion took place 3 to 4 hours after exposure was discontinued; the half life for elimination was 23.6 hours. It was found that after physical exercise, a second maximum of excretion was observed approximately 3 hours after the first one. Approximately 22% of the absorbed ethylene glycol monoethyl ether acetate dose was recovered in the urine as ethoxyacetic acid after 42 hours.
来源:Hazardous Substances Data Bank (HSDB)
代谢
对一组五名女性在正常生产期间5天以及生产停止后12天的7天内,每天暴露于乙二醇乙醚和乙二醇乙醚醋酸酯的情况下的乙氧基乙酸尿液排泄进行了研究。乙二醇乙醚和乙二醇乙醚醋酸酯的平均联合暴露浓度(以乙二醇乙醚的等效重量表示)为14.0 mg/立方米,偶尔略高于当前比利时职业暴露限值。在第一个观察期间,乙二醇乙醚和乙二醇乙醚醋酸酯的每日联合暴露曲线相对稳定,但在最后一周趋于下降。在工作周期间,尿液中乙氧基乙酸的排泄明显增加。在周末,消除远未完成,即使在12天的非暴露期后,仍可检测到代谢物的痕迹。根据第一个周期的观察结果,发现5天内平均暴露量(914.4 mg/立方米)与周末乙氧基乙酸排泄量(105.7 mg/g肌酐)之间存在良好的线性相关性。发现乙氧基乙酸的估计值为150 ± 35 mg/g,与重复5天全班次暴露于乙二醇乙醚(19 mg/立方米)或乙二醇乙醚醋酸酯(27 mg/立方米)的相应职业暴露限值相对应。
The urinary excretion of ethoxyacetic acid was studied in a group of five women daily exposed to the ethyl ether of ethylene glycol and the ethyl ether of ethylene glycol acetate during 5 days of normal production and 7 days after a 12 days production stop. The mean combined exposure concentration of ethyl ether of ethylene glycol and ethylene glycol ethyl ether acetate (expressed in equivalent weight of ethyl ether of ethylene glycol was 14.0 mg/cu m with occasional slight excursions above the current Belgian occupational exposure limit. The daily combined exposures profiles for ethylene glycol ethyl ether and ethylene glycol ethyl ether acetate were rather constant during the first observation period, but they tended to decrease during the last week. The urinary ethoxyacetic acid excretion clearly increased during the work week. Over the weekends the elimination was far from complete, and even after a prolonged nonexposure period of 12 days traces of the metabolite were still detectable. Based on the observations from the first period, a good linear correlation was found between the average exposure over 5 days (914.4 mg/cu m) and the ethoxyacetic acid excretion of the end of the week (105.7 mg/g creatinine). An ethoxyacetic acid estimate of 150 + or - 35 mg/g was found to correspond with repeated 5 days full-shift exposures to the respective occupational exposure limit of ethylene glyol ethyl ether (19 mg/cu m) or ethylene glycol ethyl ether acetate (27 mg/cu m).
来源:Hazardous Substances Data Bank (HSDB)
代谢
在一家油漆生产厂,有十七名接触了乙二醇醚的工人接受了外部和内部溶剂暴露的检查。生产厂的工人(n=12)接触到的平均浓度的乙氧基乙醇、乙氧基乙醋酸酯、丁氧基乙醇、1-甲氧基丙醇-2、2-甲氧基丙基-1-醋酸酯和二甲苯分别为2.8;2.7;1.1;7.0;2.8和1.7 ppm。通过测量血液中的丁氧基乙醇以及尿液样本中的乙氧基醋酸和丁氧基醋酸来估计内部暴露。正如预期的那样,在油漆生产中发现了最高的值。换班后的丁氧基乙醇、乙氧基醋酸和丁氧基醋酸的平均浓度分别为121.3 ug/L;167.8和10.5 mg/L。换班前样本中乙氧基醋酸和丁氧基醋酸的相对较高浓度可以用这些代谢物较长的半衰期来解释。大多数乙二醇醚是通过皮肤吸收的。作者认为,未来尿液中乙氧基醋酸浓度的可容忍限值应在100到200 mg/L的范围内。
Seventeen persons who were exposed to glycol ethers in a varnish production plant, were examined according to their external and internal solvent exposure. The workers in the production plant (n= 12) were exposed to average concentrations of ethoxyethanol, ethoxyethyl acetate, butoxyethanol, 1-methoxypropanol-2, 2-methoxypropyl-1-acetate and xylene of 2.8; 2.7; 1.1; 7.0; 2.8 and 1.7 ppm. Internal exposure was estimated by measuring butoxyethanol in blood as well as ethoxyacetic acid and butoxyacetic acid in urine samples. As expected, the highest values were found in the varnish production. The average post shift concentrations of butoxyethanol, ethoxyacetic acid and butoxyacetic acid were 121.3 ug/L; 167.8 and 10.5 mg/L. The relatively high concentrations of ethoxyacetic acid and butoxyacetic acid in pre-shift samples can be explained by the long half-lives of these metabolites. Most of the glycol ethers were taken up through the skin. The authors think that a future tolerable limit value for the concentration of ethoxyacetic acid in urine should be in the order of 100 to 200 mg/L.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
乙二醇的甲基和乙基醚,即2-甲氧基乙醇(2-ME)酯、2-甲氧基乙酯(2-MEA)和2-乙氧基乙酯(2-EEA)...都是稳定的,并且都能与(或对于2-EEA来说,在)水中混溶,并且与大量的有机溶剂混溶。...这些醇醚在水中的溶解性和它们相对较低的蒸气压可能会导致在没有降解的情况下在水体中积累。然而,土壤、污泥和水体中的微生物降解似乎阻止了这种可能性。由于使用醇醚作为挥发性溶剂而导致的大气排放是最大的环境暴露途径....对环境生物的影响:2-ME和2-EE对微生物和水生动物的毒性似乎很低。...醇醚醋酸酯(2-MEA和2-EEA)对鱼类的毒性要大得多。2-EEA对拟鲤的LC50为46毫克/升,2-MEA对食蚊鱼和蓝鳃的LC50为45毫克/升。...对实验动物和体外测试系统的影响:系统毒性:2-ME和2-EE对实验动物的毒性研究比2-MEA和2-EEA要广泛得多。2-ME和2-EE及其醋酸酯在单次暴露后具有相似的致死性,并且无论是通过皮肤、口服还是吸入途径,都表现出较低的急性致死性。口服LD60值对于多种物种的范围为2-ME为900至3400毫克/千克体重,2-EE为1400至5500毫克/千克,2-MEA为1250至3930毫克/千克,2-EEA为1300至5100毫克/千克。...2-EE被发现具有致畸性(在轻微母体毒性存在的情况下)。另一项研究报告称,在大鼠暴露于184或920毫克/立方米2-EE和家兔暴露于644毫克/立方米2-EE的情况下,出现了胎儿毒性但没有畸形。发育影响的NOEL值为大鼠37毫克/立方米,家兔184毫克/立方米。在大鼠怀孕的第7-13天或第14-20天暴露于368毫克/立方米2-EE的后代中,观察到了行为和神经化学变化。在大鼠怀孕的第7-16天,每天四次涂抹0.25毫升未稀释的2-EE(四次/天)的处理组,在未出现母体毒性的情况下,表现出了明显的胎儿毒性和高畸形发生率。在相同方案下,使用等摩尔剂量的2-EEA处理大鼠,也观察到了类似的效果(0.35毫升,四次/天)。在大鼠怀孕的第6-18天,将家兔暴露于2-EEA的吸入剂量,在两个不同的研究中,在2176毫克/立方米和544毫克/立方米的剂量下产生了致畸反应,这两个研究的发育NOEL值分别为135毫克/立方米和270毫克/立方米。在大鼠怀孕的第6-15天,将大鼠暴露于2-EEA,在540毫克/立方米的剂量下产生了胎儿毒性,在1080毫克/立方米的剂量下产生了畸形。发育的NOEL值为170毫克/立方米。对人类的影响:关于这四种醇醚对人类的毒性影响的信息有限。从少数案例报告和职业流行病学研究的结果与在实验动物中看到的负面效果一致。没有找到量化一般人群暴露和健康影响报告。
... The methyl and ethyl ethers of ethylene glycol, ie 2- methoxyethanol (2-ME) esters, 2-methoxyethyl acetate (2-MEA) and 2-ethoxyethyl acetate (2- EEA) ... are all stable, and are all miscible with (or in the case of 2-EEA very soluble in) water and miscible with a large number of organic solvents. ... The solubility of these glycol ethers in water and their relatively low vapor pressure could result in their build-up in water in the absence of degradation. However, degradation by microorganisms in soil, sewage sludge, and water appears to prevent this possibility. Atmospheric emissions resulting from the use of glycol ethers as evaporative solvents result in the greatest environmental exposure.... Effects on Organisms in the Environment: The toxicity of 2-ME and 2-EE to microorganisms and aquatic animals appears to be low. ... The glycol ether acetates (2-MEA and 2-EEA) are far more toxic to fish. The LC50 of 2-EEA for fathead minnows is 46 mg/L and that of 2-MEA for tidewater silverfish and bluegills is 45 mg/L. ... Effects on Experimental Animals and In Vitro Test Systems: Systemic toxicity: The toxicity of 2-ME and 2-EE to experimental animals has been much more widely studied than that of 2-MEA and 2-EEA. 2-ME and 2-EE and their acetates have similar lethalities after single exposures and they show low acute lethality whether exposure is via the dermal, oral, or inhalation route. Oral LD60 values for a variety of species range between 900 and 3400 mg/kg body weight for 2-ME, 1400 and 5500 mg/kg for 2-EE, 1250 and 3930 mg/kg for 2-MEA, and 1300 and 5100 mg/kg for 2-EEA. ... 2-EE was found to be teratogenic (in the presence of slight maternal toxicity). Another study reported fetotoxicity but no malformations in rats exposed to 184 or 920 mg 2-EE/cu m, and in rabbits exposed to 644 mg 2-EE/cu m. NOEL values for developmental effects were 37 mg/cu m for rats and 184 mg/cu m for rabbits. Behavioral and neurochemical alterations were seen in the offspring of rats exposed to 368 mg 2-EE/cu m on days 7-13 or 14-20 of gestation. Rats treated by dermal application of 0.25 mL undiluted 2-EE (four times daily on gestation days 7-16) exhibited marked fetotoxicity and a high incidence of malformation in the absence of maternal toxicity. Similar effects were noted following 2-EEA treatment of rats, using the same protocol, at an equimolar dose (0.35 mL, four times daily). Inhalation exposure of rabbits to 2-EEA on gestation days 6-18 produced teratogenic responses at 2176 mg/cu m and 544 mg/cu m in two different studies, the developmental NOEL values in these two studies being 135 mg/m3 and 270 mg/m3. Exposure of rats to 2-EEA on days 6-15 of gestation produced fetotoxicity at 540 mg/cu m and malformation at 1080 mg/cu m. The developmental NOEL was 170 mg/cu m. Effects on humans: Information on the toxic effects of these four glycol ethers on humans is limited. The results from the few case reports and workplace epidemiological studies are consistent with the adverse effects seen in experimental animals. No reports quantifying general population exposure and health effects have been found. ...
来源:Hazardous Substances Data Bank (HSDB)
毒理性
该物质可以通过吸入其蒸汽、通过皮肤接触以及摄入进入人体。
The substance can be absorbed into the body by inhalation of its vapour, through the skin and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
吸入,皮肤吸收,吞食,皮肤和/或眼睛接触
inhalation, skin absorption, ingestion, skin and/or eye contact
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眼睛、鼻子刺激;呕吐;肾脏损害;麻痹;在动物中:生殖毒性,致畸效应。
irritation eyes, nose; vomiting; kidney damage; paralysis; In Animals: reproductive, teratogenic effects
来源:The National Institute for Occupational Safety and Health (NIOSH)
毒理性
眩晕。嗜睡。头痛。昏迷。
Dizziness. Drowsiness. Headache. Unconsciousness.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
吸收、分配和排泄
...不易被皮肤吸收,但经长时间大量使用后,有毒物质可能被吸收。
... Not readily absorbed by skin, but with intensive prolonged application toxic amount can be absorbed.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
... Once absorbed into body, esters are saponified and systemic effect is quite typical of parent glycol or glycol ether. /Ether-esters of glycols/
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
研究了人体对乙酸乙二醇单乙醚的肺部吸收和消除。男性志愿者在静息或运动状态下接触。暴露期为4小时,每小时的暴露之间有10分钟的休息时间。随着暴露的进行,乙酸乙二醇单乙醚的保留、大气清除和吸收速率降低。这些参数的稳态值在3到4小时后达到。随着吸收的增加,呼出气体中乙酸乙二醇单乙醚的相对量减少。个体对乙酸乙二醇单乙醚的吸收主要受肺部通气率、心输出量和人体测量因素(如体脂百分比)的支配。保留随着乙酸乙二醇单乙醚暴露浓度的增加和运动期间工作负荷的增加而增加。吸收速率也随着运动期间工作负荷的增加而增加。这主要归因于肺部通气率的增加。在呼出气体中检测到乙酸乙二醇单乙醚以及乙酸乙二醇单乙醚。暴露结束后,乙酸乙二醇单乙醚的呼吸消除是双相的。然而,乙酸乙二醇单乙醚醋酸通过呼吸消除是排泄的次要途径,只有大约0.5%的吸收在呼出气体中恢复。乙酸乙二醇单乙醚醋酸的呼吸消除仅与总摄取量、体脂含量和肺通气率显著相关。
Pulmonary absorption and elimination of ethylene glycol monoethyl ether acetate were studied in humans. Male volunteers were exposed at rest or while exercising. Exposure periods were 4 hr with a 10 min break between each hour of exposure. ... Retention, atmospheric clearance, and rate of uptake of ethylene glycol monoethyl ether acetate decreased as exposure proceeded. Steady state values of these parameters were attained after 3 to 4 hr. The relative amounts of ethylene glycol monoethyl ether acetate in the expired air decreased as uptake increased. Individual uptake of ethylene glycol monoethyl ether acetate was mainly governed by pulmonary ventilation rate, cardiac output and anthropometric factors such as percent body fat. Retention increased with increasing ethylene glycol monoethyl ether acetate exposure concentration and increasing workload during exercise. The rate of uptake also increased with workload during exercise. This was attributed primarily to an increased pulmonary ventilation rate. Ethylene glycol monoethyl ether as well as ethylene glycol monoethyl ether acetate was detected in the expired air. Respiratory elimination of ethylene glycol monoethyl ether after exposure ended was biphasic. Respiratory elimination of ethylene glycol monoethyl ether acetate, however, was a minor route of excretion, only around 0.5% uptake being recovered in the expired air. Respiratory elimination of ethylene glycol monoethyl ether acetate was significantly correlated only with total body uptake, body fat content, and pulmonary ventilation rate.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
在雄性比格犬中,通过吸入、皮肤应用和静脉给药比较了2-丙氧基乙酯和2-乙氧基乙酯的吸收和消除速率。暴露于50 ppm的2-丙氧基乙酯和2-乙氧基乙酯导致它们通过肺部迅速吸收。随着暴露时间的延长,2-丙氧基乙酯和2-乙氧基乙酯的呼吸浓度迅速升高,并在3小时后达到平台期。在静脉给药1 mg/kg的放射性标记2-丙氧基乙酯后,犬在24小时内通过尿液排除了约88%的放射性;约61%的等效剂量的2-乙氧基乙酯在24小时内通过尿液排出。2-丙氧基乙酯给药后的血液放射性清除速度比2-乙氧基乙酯快。对于每种化合物,30分钟和60分钟暴露后吸收的量相似。2-丙氧基乙酯和2-乙氧基乙酯的体外经皮吸收滞后时间分别为1.2小时和1.6小时。2-乙氧基乙酯和2-丙氧基乙酯均未改变皮肤制剂的扩散特性。尽管2-丙氧基乙酯和2-乙氧基乙酯的排泄率明显不同,但通过皮肤和肺部吸收的每种化合物的总量相似。
The absorption and elimination rates of 2-propoxyethyl acetate and 2-ethoxyethyl acetate were compared following inhalation, dermal application, and intravenous administration in male beagle-dogs. Exposure to 50 ppm 2-propoxyethyl acetate and 2-ethoxyethyl acetate resulted in rapid absorption through the lungs. Breath concentrations of 2-propoxyethyl acetate and 2-ethoxyethyl acetate showed a rapid increase with the duration of the exposure and reached a plateau after 3 hours. After iv administration of 1 mg/kg radiolabeled 2-propoxyethyl acetate, dogs eliminated about 88% of the radioactivity in the urine within 24 hours; about 61% of an equivalent dose of 2-ethoxyethyl acetate was excreted in the urine within 24 hours. Blood radioactivity after 2-propoxyethyl acetate administration was cleared more rapidly than after 2-ethoxyethyl acetate. For each compound, the amounts absorbed were similar after 30 and 60 minute exposures. The in vitro percutaneous absorption lag times were 1.2 and 1.6 hours for 2-propoxyethyl acetate and 2-ethoxyethyl acetate, respectively. Neither 2-ethoxyethyl acetate nor 2-propoxyethyl acetate altered the diffusion properties of the skin preparations. Although the excretion rates for 2-propoxyethyl acetate and 2-ethoxyethyl acetate are markedly different, the total amount of each compound absorbed through the skin and lungs is similar.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
职业暴露等级:B
-
职业暴露限值:TWA: 0.5 ppm (2.7 mg/m3) [skin]
-
TSCA:Yes
-
危险等级:3
-
立即威胁生命和健康浓度:500 ppm
-
危险品标志:T
-
安全说明:S45,S53
-
危险类别码:R60,R20/21/22,R10,R61
-
WGK Germany:1
-
海关编码:2915390090
-
危险品运输编号:UN 1172 3/PG 3
-
RTECS号:KK8225000
-
包装等级:III
-
危险类别:3
-
储存条件:储存于阴凉、通风的库房。远离火种、热源,库温不宜超过37℃。保持容器密封,并与氧化剂、酸类、碱类分开存放,切忌混储。应采用防爆型照明和通风设施,禁止使用易产生火花的机械设备和工具。储区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
化学性质
无色液体。凝固点-61.7℃,沸点156.3℃,51℃(2.8kPa),相对密度0.973(20℃),熔点-61.7℃,折射率1.4055(20℃),闪点51℃(闭杯),66℃(开杯),燃点379℃。它能与大多数有机溶剂混溶,并可溶解于水中。此外,该物质还散发出一种令人愉悦的酯类香气。
用途
乙二醇乙醚醋酸酯可用作树脂、皮革、油墨等材料的溶剂,还可与其他化合物配合使用,例如作为皮革粘合剂、油漆剥离剂及金属热镀抗腐蚀涂料等。
另外,它也可用于金属和家具喷漆的溶剂以及刷涂漆用溶剂。此外,乙二醇乙醚醋酸酯还可作为保护性涂料、染料、树脂、皮革及油墨的溶剂,并可用于金属、玻璃及其他硬表面清洗剂的配方中。同时,此物质亦可作为一种优良的化学试剂。
生产方法
- 通过将乙酐和浓硫酸混合加热至130℃后,慢慢滴加乙二醇单乙醚,在反应温度保持在130-135℃时继续滴加并维持流动1-2小时。然后回流温度为140℃。冷却后用碳酸钠中和pH值至7-8,再以工业无水碳酸钾干燥。滤出干燥剂进行粗分馏,收集150-160℃间的馏出物。再分馏,收集155.5-156.5℃的馏分即为成品。
- 也可由乙二醇单乙醚与乙酸在苯中以浓硫酸催化下回流反应得到。
- 在阳离子树脂催化下,乙二醇单乙醚与乙酸反应,不断除去生成的水,然后进行蒸馏即可得到产品。此法乙酸转化率为94.5%,产品的选择性为98.5%。
- 2-乙氧基乙酸与乙醇反应也可得之。
- 醋酸乙酯与乙氧基乙醇进行酯交换反应,或者醋酸直接与乙氧基乙醇反应亦可得到产物。最后,环氧乙烷和醋酸乙酯的反应也是另一种生产方法。
类别
易燃液体
毒性分级
中毒
急性毒性
口服- 大鼠 LD50: 2700 毫克/ 公斤;小鼠 LD50: 1910 毫克/ 公斤
刺激数据
皮肤接触 - 兔子 490 毫克,轻度刺激;眼睛接触 - 兔子 40 毫克,中度刺激。
可燃性危险特性
遇明火、高温或氧化剂时易燃烧,并产生刺激烟雾。
储运特性
库房应保持通风、低温和干燥。避免与氧化剂分开存放。
职业标准
TWA 27 毫克/立方米;STEL 50 毫克/立方米
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 乙二醇甲醚乙酸酯 2-methoxyethyl acetate 110-49-6 C5H10O3 118.133 乙酸乙酯 ethyl acetate 141-78-6 C4H8O2 88.1063 乙酸酐 acetic anhydride 108-24-7 C4H6O3 102.09 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 乙二醇二乙酸酯 ethylene glycol diacetate 111-55-7 C6H10O4 146.143 1,2-乙二基 1-乙酸酯 2-甲酸酯 1,2-ethanediol acetate formate 29776-97-4 C5H8O4 132.116
反应信息
-
作为反应物:描述:乙二醇乙醚醋酸酯 在 氘代丙酮 、 potassium carbonate 、 氯化(1-丁基-3-甲基咪唑) 作用下, 反应 12.0h, 以72%的产率得到deuterated 2-ethoxyethyl acetate参考文献:名称:咪唑阳离子与酮的动态共价相互作用诱导的有机催化氘化。摘要:在本文中,我们提出了一种基于咪唑鎓阳离子与酮的动态共价相互作用的新的有机催化方法。N-烷基咪唑鎓盐与丙酮d 6在含氧碱的存在下反应生成动态有机催化体系,质子化的卡宾/酮加合物作为H / D交换催化剂。已开发的pH依赖性氘代方法显示出较高的标记选择性和良好的手性官能团耐受性。在这里,我们报告了一种有效的无金属氘化的独特方法,该方法可以标记各种类型的α-酸性化合物而不会造成痕量金属污染。DOI:10.1002/adsc.202001507
-
作为产物:参考文献:名称:Alder,K. et al., Chemische Berichte, 1961, vol. 94, p. 1860 - 1867摘要:DOI:
-
作为试剂:参考文献:名称:Light sensitive photographic material containing a mordant layer摘要:适用于染料扩散转移过程中的彩色照相材料图像接收层的媒染聚合物是一种含有环氧基的水溶性阳离子聚合物。这种聚合物可以通过用能够引入环氧基的季铵化剂季铵化公式为##STR1##的碱性聚氨酯、聚脲或聚脲聚氨酯制备而成。重复单元包含可季铵化的三级氨基团,重复单元B来源于双氯甲酸酯或二异氰酸酯。公开号:US04186014A1
文献信息
-
BIS(ARYLMETHYLIDENE)ACETONE COMPOUND, ANTI-CANCER AGENT, CARCINOGENESIS-PREVENTIVE AGENT, INHIBITOR OF EXPRESSION OF Ki-Ras, ErbB2, c-Myc AND CYCLINE D1, BETA-CATENIN-DEGRADING AGENT, AND p53 EXPRESSION ENHANCER申请人:Shibata Hiroyuki公开号:US20100152493A1公开(公告)日:2010-06-17It has been demanded to improve the poor solubility of curcumin to develop an anti-tumor compound capable of inhibiting the growth of various cancer cells at a low concentration. Thus, disclosed is a novel synthetic compound, a bis(arylmethylidene)acetone, which has both of an excellent anti-tumor activity and a chemo-preventive activity. A bis(arylmethylidene)acetone (i.e., a derivative having a curcumin skeleton) which is an anti-tumor compound and has a chemo-preventive activity is synthesized and screened. A derivative having enhanced anti-tumor activity and chemo-preventive activity can be synthesized.
-
[EN] OXIME ESTER PHOTOINITIATORS<br/>[FR] PHOTO-INITIATEURS À BASE D'ESTER D'OXIME申请人:BASF SE公开号:WO2021175855A1公开(公告)日:2021-09-10Disclosed are α-oxo oxime ester compounds based on carbazole derivatives which have specific substituent groups useful as a photoinitiator, as well as photopolymerizable compositions comprising said photoinitiator and ethylenically unsaturated compounds. The photopolymerizable compositions are useful, for example, in photoresist formulations for display applications, e.g. liquid crystal display (LCD), organic light emitting diode (OLED) and touch panel.
-
Method of Fabricating Glycol Monoalkyl Ether Acetate Using Acidic Ionic Liquid Catalyst申请人:Wu Jung-Chung公开号:US20110184207A1公开(公告)日:2011-07-28A new method for fabricating glycol monoalkyl ether acetate (GMAEA) is provided. A Bronsted acidic ionic liquid is used. After some reactions, two layers of materials are formed. A product of GMAEA is obtained at the upper layer. The lower layer is the ionic liquid. Thus, the ionic liquid is reusable for re-fabricating the product. And, furthermore, waste acid is reduced.
-
AROMATIC AMINE COMPOUND, CURING AGENT FOR EPOXY COMPOUND, CURABLE COMPOSITION, CURED PRODUCT, METHOD FOR PRODUCING CURED PRODUCT, AND METHOD FOR PRODUCING AROMATIC AMINE COMPOUND申请人:TOKYO OHKA KOGYO CO., LTD.公开号:US20210130284A1公开(公告)日:2021-05-06An aromatic amine compound capable of satisfactorily forming a cured product having exceptional alkali resistance by reaction with an epoxy compound; a curing agent for an epoxy compound, the curing agent including the aromatic amine compound; a curable composition including the curing agent for an epoxy compound; a cured product of the curable composition; a method for producing the cured product; and a method for producing the abovementioned aromatic amine compound. The aromatic amine compound has a structure such that a specific position in a central skeleton comprising a fused ring such as a fluorene ring is substituted with a side-chain group including two aromatic groups linked by a flexible bond such as an amide bond, at least one amino group is bonded to the end of the side-chain group, and the structure has no hydroxyl groups.
-
ANTIREFLECTIVE COMPOSITIONS WITH THERMAL ACID GENERATORS申请人:Rohm and Haas Electronic Materials Korea Ltd.公开号:US20190085173A1公开(公告)日:2019-03-21New methods and substrates are provided that include antireflective compositions that comprise one or more thermal acid generators.提供了包括一个或多个热酸发生剂的防反射组分的新方法和基板。
表征谱图
-
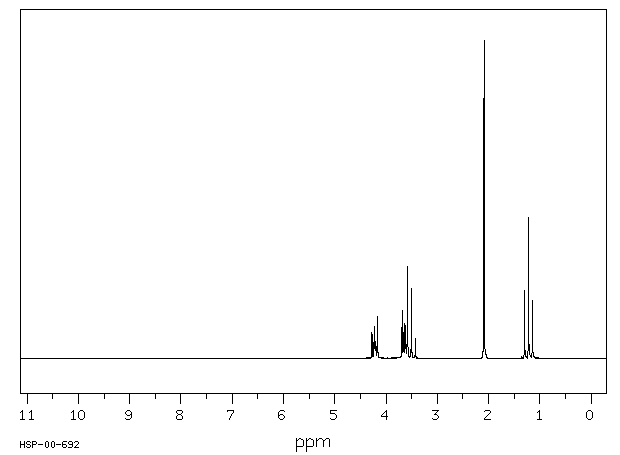
氢谱1HNMR
-
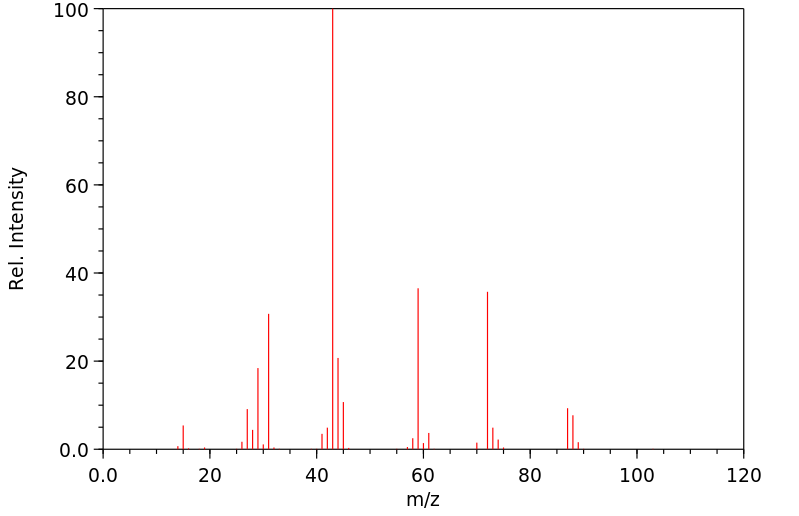
质谱MS
-
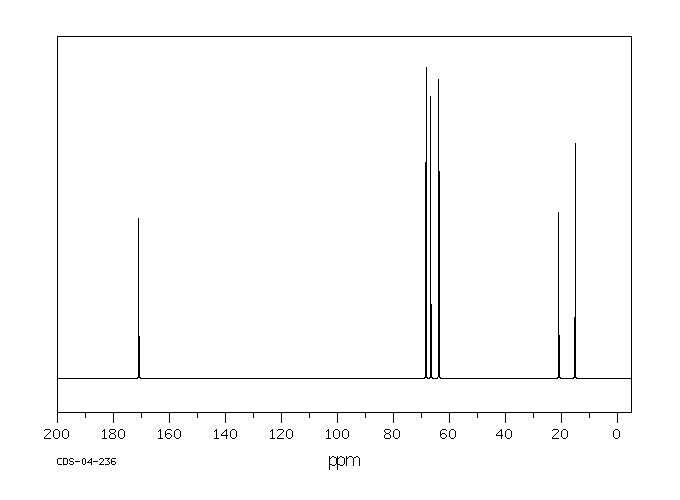
碳谱13CNMR
-
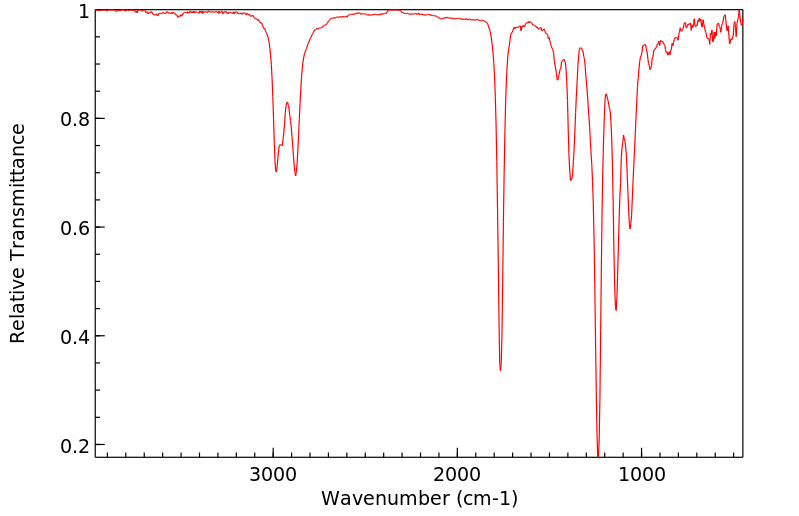
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸