邻苯基苯酚缩水甘油醚 | 7144-65-2
物质功能分类
中文名称
邻苯基苯酚缩水甘油醚
中文别名
2-苯基苯酚缩水甘油醚;2-联苯缩水甘油醚;3-(2-联苯)-1,2-环氧丙烷
英文名称
2-(([1,1'-biphenyl]-2-yloxy)methyl)oxirane
英文别名
2-((biphenyl-2-yloxy)methyl)oxirane;2-(([1,1′-biphenyl]-2-yloxy)methyl)oxirane;2-Biphenylyl glycidyl ether;2-[(2-phenylphenoxy)methyl]oxirane
CAS
7144-65-2
化学式
C15H14O2
mdl
MFCD00046989
分子量
226.275
InChiKey
DNVXWIINBUTFEP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:31°C
-
沸点:200°C/13mmHg(lit.)
-
密度:1.0891 (rough estimate)
-
稳定性/保质期:
遵照规格使用和储存则不会分解。
计算性质
-
辛醇/水分配系数(LogP):3.2
-
重原子数:17
-
可旋转键数:4
-
环数:3.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:21.8
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
RTECS号:SL7800000
-
海关编码:2910900090
-
储存条件:请将密封于阴凉干燥处。
SDS
| Name: | 2-Biphenylyl Glycidyl Ether 95% Material Safety Data Sheet |
| Synonym: | 3-(2-Biphenylyloxy)-1,2-Epoxypropane; Ether, 2-Biphenyl 2,3-epoxypropyl |
| CAS: | 7144-65-2 |
Synonym:3-(2-Biphenylyloxy)-1,2-Epoxypropane; Ether, 2-Biphenyl 2,3-epoxypropyl
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7144-65-2 | 2-Biphenylyl Glycidyl Ether | 95% | 230-451-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If breathing is difficult, give oxygen. Get medical aid. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits.
Exposure Limits CAS# 7144-65-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Solid
Color: white
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 120 deg C @ .10mmHg
Freezing/Melting Point: 30.00 - 32.00 deg C
Autoignition Temperature: Not applicable.
Flash Point: Not applicable.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C15H14O2
Molecular Weight: 226.27
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents, strong acids, strong bases.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7144-65-2: RR0356000 LD50/LC50:
Not available.
Carcinogenicity:
2-Biphenylyl Glycidyl Ether - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 7144-65-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7144-65-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7144-65-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 3- -2-hydroxy-propylchlorid 20804-51-7 C15H15ClO2 262.736 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-([1,1'-biphenyl]-2-yloxy)propan-2-ol 33356-01-3 C15H16O2 228.291 —— 1-(biphenyl-2-yloxy)-3-(prop-2-ynylamino)propan-2-ol 1453205-17-8 C18H19NO2 281.354 —— 3-(biphenyl-2-yloxy)-2-hydroxypropyl acrylate 91442-28-3 C18H18O4 298.339 柏拉非农 Berlafenone hydrochloride 18965-97-4 C19H25NO2 299.413 —— 1-(cyclopropylamino)-3-(2-phenylphenoxy)propan-2-ol 1096982-65-8 C18H21NO2 283.37 —— 1-(biphenyl-2-yloxy)-3-(methyl(prop-2-inyl)amino)propan-2-ol 1453205-18-9 C19H21NO2 295.381 —— 1-(N-isopropyl-N-methylamino)-3-(2-phenylphenoxy)propan-2-ol 63638-03-9 C19H25NO2 299.413
反应信息
-
作为反应物:描述:邻苯基苯酚缩水甘油醚 在 diisobutylaluminum borohydride 作用下, 以 四氢呋喃 为溶剂, 反应 1.0h, 以90%的产率得到1-([1,1'-biphenyl]-2-yloxy)propan-2-ol参考文献:名称:二氢化硼二异丁基铝氢化物与含有代表性官能团的某些有机化合物的反应摘要:由氢化二异丁基铝(DIBAL)和硼烷二甲基硫醚(BMS)合成的二元氢化物二异丁基硼氢化铝[(i Bu)2 AlBH 4 ]在还原各种有机官能团方面显示出巨大潜力。这种独特的二元氢化物(i Bu)2 AlBH 4易于合成,通用且易于使用。醛,酮,酯和环氧化物以基本定量的收率非常快地还原为相应的醇。该二元氢化物可以在25°C下以有效方式将叔酰胺迅速还原为相应的胺。此外,腈以基本上定量的产率转化为相应的胺。这些反应在环境条件下发生,并在一个小时或更短的时间内完成。还原产物可通过简单的酸碱萃取而分离,无需使用柱色谱法。进一步的研究表明(i Bu)2 AlBH 4如一系列竞争反应所示,它具有成为选择性氢化物供体的潜力。讨论了(i Bu)2 AlBH 4,DIBAL和BMS之间的异同。DOI:10.1021/acs.joc.0c03062
-
作为产物:描述:参考文献:名称:1-(Trialkylamino)-3-(phenylphenoxy)-2-propanol quarternary salts摘要:上述标题化合物表现出强大且长效的抗心律失常活性,同时缺乏相关先前技术化合物特征的不良副作用,例如β-肾上腺能受体阻滞活性和局部麻醉活性。公开号:US04241088A1
文献信息
-
Ruthenium-Catalyzed Selective Hydrogenation of Epoxides to Secondary Alcohols作者:Subramanian Thiyagarajan、Chidambaram GunanathanDOI:10.1021/acs.orglett.9b03995日期:2019.12.6A ruthenium(II)-catalyzed highly selective Markovnikov hydrogenation of terminal epoxides to secondary alcohols is reported. Diverse substitutions on the aryl ring of styrene oxides are tolerated. Benzylic, glycidyl, and aliphatic epoxides as well as diepoxides also underwent facile hydrogenation to provide secondary alcohols with exclusive selectivity. Metal-ligand cooperation-mediated ruthenium trans-dihydride
-
AROMATIC GLYCOL ETHERS AS WRITING MONOMERS IN HOLOGRAPHIC PHOTOPOLYMER FORMULATIONS申请人:COVESTRO DEUTSCHLAND AG公开号:US20170045816A1公开(公告)日:2017-02-16The invention relates to a photopolymer formulation comprising specific aromatic glycol ethers as writing monomers, matrix polymers and a photoinitiator. The invention further provides an unexposed holographic medium obtainable using an inventive photopolymer formulation, and an exposed holographic medium obtainable by exposing a hologram into an inventive unexposed holographic medium. The invention likewise provides a visual display comprising an inventive exposed holographic medium, for the use of an inventive exposed holographic medium for production of chip cards, identification documents, 3D images, product protection labels, labels, banknotes or holographic optical elements, and specific aromatic glycol ethers.
-
High yield room temperature conversion of carbon dioxide into cyclic carbonates catalyzed by mixed metal oxide (CuO‐ZnO) nano‐flakes/micro‐flakes (Cozi‐nmf)作者:Venkateswara Rao Velpuri、Krishnamurthi MuralidharanDOI:10.1002/aoc.6224日期:2021.6Capturing and converting carbon dioxide (CO2) into useful organic molecules and polymers is the best way of alleviating excessive release of it from industrial sources to the environment. Cyclic carbonate synthesis by cycloaddition from CO2 and epoxides through a catalytic process is a 100% atom economic reaction established five decades ago. Despite the availability of many efficient catalytic systems捕获并将二氧化碳(CO 2)转化为有用的有机分子和聚合物是减轻二氧化碳从工业来源向环境中过度释放的最佳方法。通过催化过程由CO 2和环氧化物进行环加成合成环碳酸酯是五十年前建立的100%原子经济反应。尽管有许多有效的催化系统可用,但是在乏味的催化剂制备过程中,与均相催化有关的分离问题或需要纯CO 2方面存在缺陷。在这项工作中,我们报告了基于铜和锌混合金属氧化物(CuO-ZnO)纳米片/微片(Cozi-nmf)的催化体系,用于CO的环加成2在室温下于无溶剂条件下使用各种环氧化物。通过简单的方法,在室温下通过研磨工艺成功地制备了新型可循环利用的非均相催化剂。本文所述的催化剂和环状碳酸酯的合成更绿色,并且使用廉价的原料生产它们。除了许多反应之外,合成的环状碳酸酯的产率超过95%。Cozi-nmf作为催化剂的高效率是基于裸露的催化剂表面的可用性来解释的,该表面有效地促进了电子的无障碍自由移动和底物的吸附。
-
Copper-catalyzed/mediated borylation reactions of epoxides with diboron reagents: access to β-hydroxyl boronic esters
-
Copper-catalyzed cross-coupling reactions of epoxides with gem-diborylmethane: access to γ-hydroxyl boronic esters
表征谱图
-
氢谱1HNMR
-
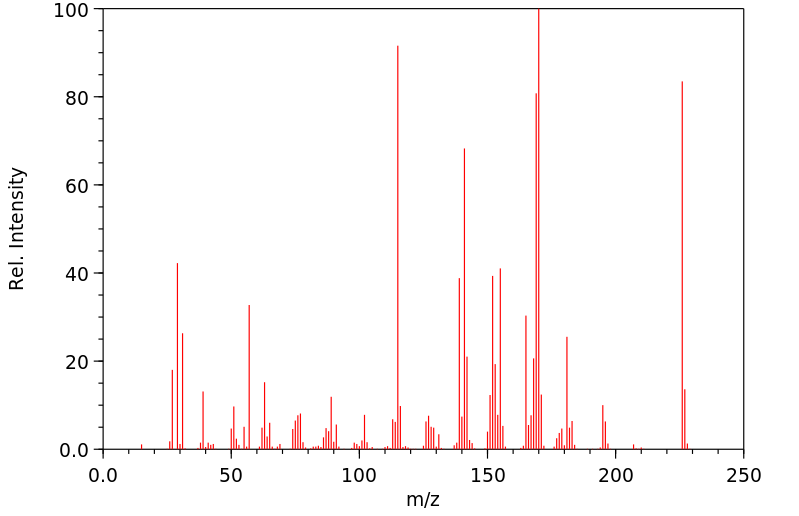
质谱MS
-
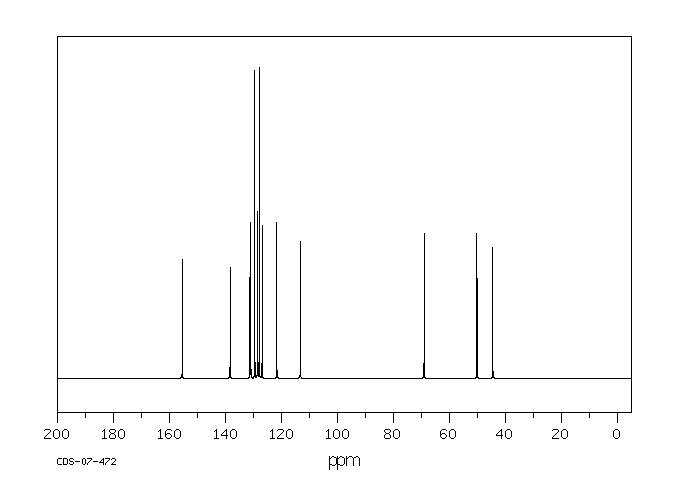
碳谱13CNMR
-
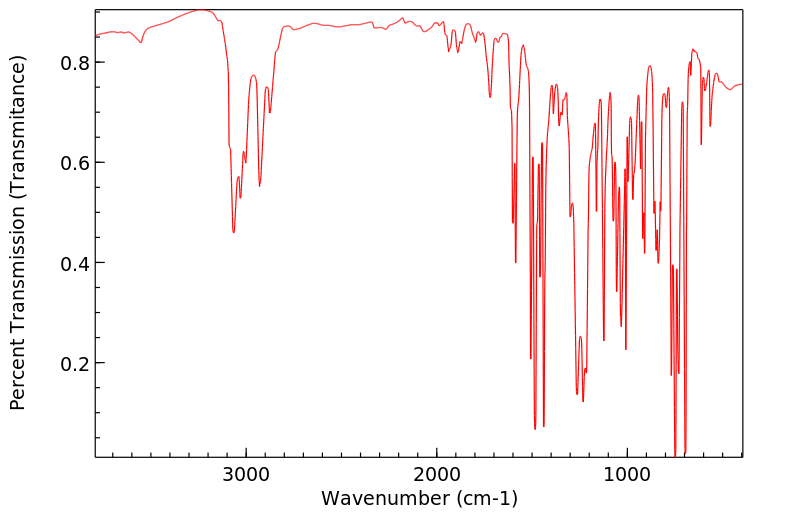
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫