卡达萘 | 483-78-3
中文名称
卡达萘
中文别名
1,6-二甲基-4-(1-甲基乙基)萘
英文名称
cadalene
英文别名
Cadalen;cadalin;1,6-dimethyl-4-(1-methylethyl)-naphthalene;4-isopropyl-1,6-dimethylnaphthalene;1.6-Dimethyl-4-isopropyl-naphthalin;1,6-dimethyl-4-propan-2-ylnaphthalene
CAS
483-78-3
化学式
C15H18
mdl
MFCD00216212
分子量
198.308
InChiKey
VMOJIHDTVZTGDO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-14.17°C (estimate)
-
沸点:bp720 291-292°; bp10 149°
-
密度:d425 0.9667
-
LogP:5.698 (est)
-
物理描述:Solid
-
保留指数:1655;1651;1682;1653;1643;1654;1660;1662;1647;1653;1662;1665;1678;1658;1673;1645;1645;1656;1654;1671;1650;1636;1660;1662;1641;1663;1654;1671;1661;1630;1641;1650;1646;1646
-
稳定性/保质期:
存在于主流烟气中。
计算性质
-
辛醇/水分配系数(LogP):5.6
-
重原子数:15
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.333
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Briggs et al., Journal of the Chemical Society, 1949, p. 1098,1100摘要:DOI:
-
作为产物:描述:参考文献:名称:Dev; Guha, Journal of the Indian Chemical Society, 1948, vol. 25, p. 13,19摘要:DOI:
文献信息
-
Autoxidation of Guaiazulene and 4,6,8-Trimethylazulene in Polar Aprotic Solvent: Structural Proof for Products作者:Yoshiharu Matsubara、Shin-ichi Takekuma、Katsumi Yokoi、Hiroshi Yamamoto、Tetsuo NozoeDOI:10.1246/bcsj.60.1415日期:1987.4Autoxidation of guaiazulene and 4,6,8-trimethylazulene at 100–120°C in DMF (or HMPA) respectively yielded 25 and 17 separable products, including several known compounds. Most of these new compounds were derivatives of 1,5- and 1,7-azulenequinone, 1H-inden-1-one, naphthoquinone, and benzenoid, or dimeric and trimeric forms; structures of these products were established on the basis of spectroscopic (NMR, UV, IR, and MS) and half-wave potential (E1⁄2) data. 1H NMR (200-MHz) parameters of various azulene derivatives are given for comparative study. Possible reaction pathways are suggested for the formation of such a wide variety of interesting products.
-
Reaction routes in catalytic reforming of poly(3-hydroxybutyrate) into renewable hydrocarbon oil作者:Shimin Kang、Jian YuDOI:10.1039/c5ra03195h日期:——ketonization of crotonic acid. The main aromatic compounds are formed in stage (3) from propylene and 2,3-dimethyl-2-cyclopenten-1-one as two key intermediates, the former from decarboxylation and the latter from ketonization of crotonic acid. The reaction routes reveal that the formation of aromatics is affected to a great extent by the concentrations of phosphoric acid and water in the reaction, which聚(3-羟基丁酸酯)或PHB是微生物的能量存储材料,可以重整为富含芳族化合物的烃油。这项工作研究了从PHB到关键中间体和最终碳氢化合物的主要反应路线。在中等温度(200–230°C)下催化磷酸的主要顺序反应包括:(1)PHB分解为巴豆酸,主要的单体中间体,(2)巴豆酸脱氧,以及(3)组合脱氧分子。PHB中的氧气以CO 2和H 2的形式除去在步骤(2)中的O,涉及巴豆酸的脱羧和酮化。主要的芳族化合物是在阶段(3)中由丙烯和2,3-二甲基-2-环戊烯-1-酮作为两个关键中间体形成的,前者来自脱羧作用,后者来自巴豆酸的酮化作用。反应路线表明,反应中磷酸和水的浓度在很大程度上影响芳烃的形成,可用于控制烃油的组成。
-
STUDIES ON THE CONSTITUENTS OF THE VOLATILE OIL FROM THE WOOD OF CHAMAECYPARIS FORMOSENSIS, MATSUM作者:Kinzo Kafuku、Nobutoshi IchikawaDOI:10.1246/bcsj.8.371日期:1933.12(i) The volatile oil from the wood of Chamaecyparis formosensis, Matsum., or Formosa cypress (benihi), contains much less terpenes and much more terpenes alcohols as compared with the volatile oil from the leaf of the same plant. (ii) The predominant terpenes in the oil are d-a-pinene and d-camphene, and they are optically strongly active. (iii) Among the terpenic alcoholic components there have been(i) 与来自同一植物叶子的挥发油相比,来自台湾 Chamaecyparis formosensis、Matsu. 或 Formosa cypress (benihi) 木材的挥发油含有更少的萜烯和更多的萜醇。(ii) 油中的主要萜烯是 da-蒎烯和 d-莰烯,它们具有很强的光学活性。(iii) 在萜烯醇成分中,已证明有两种新物质“benihiol”C10H18O 和“benihinol” 。(iv) 在倍半萜烯馏分中,一种新的倍半萜烯,其盐酸盐的熔点为 103°–104°C。被分离出来,它被命名为倍半苯。(v) 在倍半萜醇馏分中,除了卡地诺类型的叔醇外,还有至少一种伯醇,其确切性质尚不清楚。
-
The role of germacrene D as a precursor in sesquiterpene biosynthesis: investigations of acid catalyzed, photochemically and thermally induced rearrangements作者:Nils Bülow、Wilfried A KönigDOI:10.1016/s0031-9422(00)00266-1日期:2000.9Germacrene D is considered as a precursor of many sesquiterpene hydrocarbons. We have investigated the acid catalyzed as well as the photochemically and thermally induced rearrangement processes of germacrene D isolated from several Solidago species, which contain both enantiomers of germacrene D. Enantiomeric mixtures of sesquiterpenes of the cadinane, eudesmane (selinane), oppositane, axane, isodaucane
-
OXIDATION OF AZULENE DERIVATIVES. AUTOXIDATION OF GUAIAZULENE IN A POLAR APROTIC SOLVENT作者:Tetsuo Nozoe、Shin-ichi Takekuma、Masashi Doi、Yoshiharu Matsubara、Hiroshi YamamotoDOI:10.1246/cl.1984.627日期:1984.4.5Autoxidation of guaiazulene at 100 °C in N,N-dimethylformamide afforded twenty-three separable products including seven known compounds. Most of these new compounds possess highly interesting structures of azulenoquinone, inden-1-one, benzocyclobutadiene, naphthoquinone, and dimeric forms.
表征谱图
-
氢谱1HNMR
-
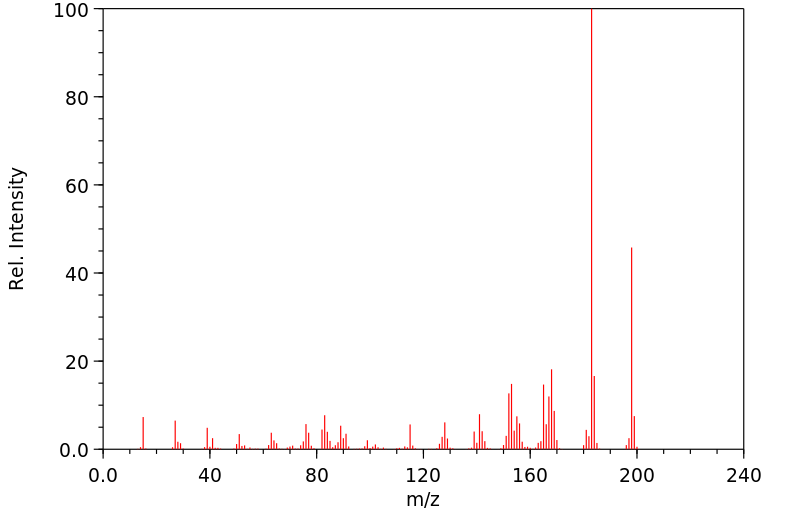
质谱MS
-
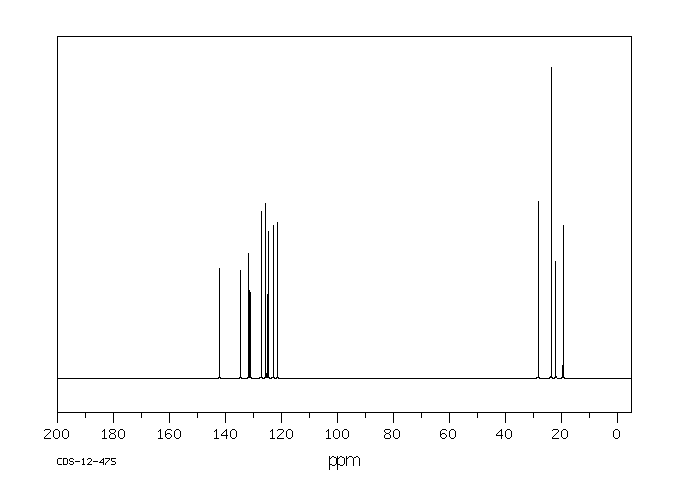
碳谱13CNMR
-
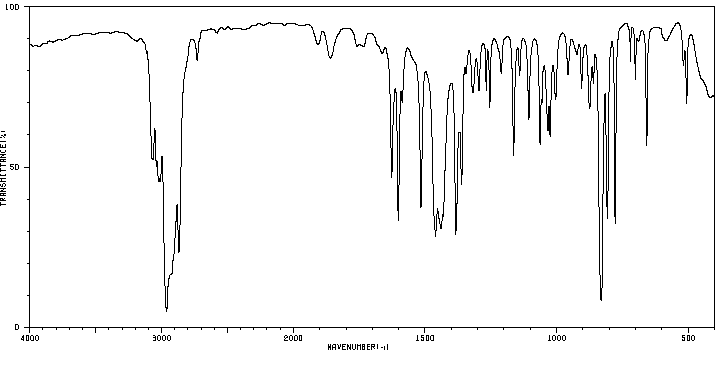
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸