吡咯酮 | 1141-06-6
中文名称
吡咯酮
中文别名
Α-吡啶偶姻
英文名称
2-hydroxy-1,2-di-2-pyridylethanone
英文别名
2-pyridoin;alpha-Pyridoin;2-hydroxy-1,2-dipyridin-2-ylethanone
CAS
1141-06-6
化学式
C12H10N2O2
mdl
MFCD00010691
分子量
214.224
InChiKey
ZKBDAJDDDOIASC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:156-160 °C(lit.)
-
沸点:354.35°C (rough estimate)
-
密度:1.2042 (rough estimate)
-
溶解度:DMSO(少量)、甲醇(少量)
-
稳定性/保质期:
常温常压下稳定,避免与强氧化剂接触。
计算性质
-
辛醇/水分配系数(LogP):0.9
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.083
-
拓扑面积:63.1
-
氢给体数:1
-
氢受体数:4
安全信息
-
危险品标志:Xi
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2933399090
-
安全说明:S26,S37/39
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | alpha-Pyridoin 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 1141-06-6 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 1141-06-6 | _-Pyridoin, 99% | 214-526-5 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.
Potential Health Effects
The toxicological properties of this material have not been investigated. Use appropriate procedures to prevent opportunities for direct contact with the skin or eyes and to prevent inhalation.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids.
Skin:
Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes.
Ingestion:
Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
In case of fire, use water, dry chemical, chemical foam, or alcohol-resistant foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Keep container closed when not in use.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate general or local exhaust ventilation to keep airborne concentrations below the permissible exposure limits. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 1141-06-6: Personal Protective Equipment Eyes: Wear safety glasses and chemical goggles if splashing is possible.
Skin:
Wear appropriate protective gloves and clothing to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Wear a NIOSH/MSHA or European Standard EN 149 approved full-facepiece airline respirator in the positive pressure mode with emergency escape provisions.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: red-brown
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: @ 760.00mm Hg
Freezing/Melting Point: 156.00 - 160.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C12H10N2O2
Molecular Weight: 214.22
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, strong oxidants.
Incompatibilities with Other Materials:
Not available.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, nitrogen.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 1141-06-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
_-Pyridoin, 99% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 1141-06-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 1141-06-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 1141-06-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 二(2-吡啶基)乙二酮 2,2'-Pyridil 492-73-9 C12H8N2O2 212.208 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,2-di-2-pyridylethanol 100142-26-5 C12H12N2O 200.24 二(2-吡啶基)乙二酮 2,2'-Pyridil 492-73-9 C12H8N2O2 212.208 1,2-二(2-吡啶基)乙二醇 1,2-di(2-pyridyl)-1,2-ethanediol 1141-05-5 C12H12N2O2 216.239
反应信息
-
作为反应物:参考文献:名称:Mathes et al., Chemische Berichte, 1951, vol. 84, p. 452,456摘要:DOI:
-
作为产物:描述:参考文献:名称:Catalytic Action of Azolium Salts. VI. Preparation of Benzoins and Acyloins by Condensation of Aldehydes Catalyzed by Azolium Salts.摘要:苯偶姻 4(在 1- 和 2-位置上带有芳基取代的 2-羟基乙酮)通过使用少量的唑盐 1 和 2 作为催化剂,由芳香醛 3 的自缩合反应高效制备得到。1,3-二甲基苯并咪唑碘盐(2)是制备乙偶姻 6(在 1- 和 2-位置上带有烷基取代的 2-羟基乙酮)的有效催化剂,通过脂肪醛 5 的自缩合反应实现。另一方面,使用 1,3-二甲基咪唑碘盐(1)催化己醛(5d)的缩合反应未能得到乙偶姻 6d,而是得到了醛醇型缩合产物 8d。DOI:10.1248/cpb.42.2633
文献信息
-
New Route to Synthesis of Unexpected Pyridoin Derivatives: Their Structure Determination with Spectroscopic and X-Ray Methods作者:Alaaddin CukurovaliDOI:10.1080/00397910902906537日期:2009.11.18new 1,2,4-triazine-3-thione derivatives were obtained by a new route using a one-step process. The structure of the compounds was determined with infrared (IR), 1H NMR, and 13C NMR spectroscopic methods and elemental analysis. A representative x-ray crystallographic analysis of 3 was given, and its x-ray data are discussed in detail. This is a facile procedure and shorter than earlier methods.
-
Chemoselective control of hydrogenation among aromatic carbonyl and benzyl alcohol derivatives using Pd/C(en) catalyst作者:Kazuyuki Hattori、Hironao Sajiki、Kosaku HirotaDOI:10.1016/s0040-4020(01)00421-5日期:2001.6of aromatic ketones and aldehydes quite smoothly give the corresponding methylene compounds via the formation of the intermediary benzyl alcohols in the presence of Pd/C as a catalyst. Therefore, it is extremely difficult to isolate the intermediary benzyl alcohol selectively. This paper describes a mild and chemoselective hydrogenation method of an aromatic carbonyl compound to benzyl alcohol using
-
CaO-catalyzed Aerobic Oxidation of α-Hydroxy Ketones: Application to One-pot Synthesis of Quinoxaline Derivatives作者:Takayoshi Hara、Yukihiro Takami、Nobuyuki Ichikuni、Shogo ShimazuDOI:10.1246/cl.2012.488日期:2012.5.5The aerobic oxidation of α-hydroxy ketones into α-diketones catalyzed by CaO is compared with the same reaction catalyzed by other metal oxides. The catalytic activities of the various metal oxides were proportional to their surface basicities. The direct conversion of α-hydroxy ketones into quinoxalines via CaO-catalyzed aerobic oxidation followed by in situ reaction with 1,2-diaminoaromatics is also achieved. Various types of quinoxalines were synthesized in the presence of the CaO catalyst and molecular oxygen. It was also found that the CaO catalyst was reusable without any loss of its catalytic activity.
-
Stetter Reaction in Room Temperature Ionic Liquids and Application to the Synthesis of Haloperidol作者:Siddam Anjaiah、Srivari Chandrasekhar、René GréeDOI:10.1002/adsc.200404123日期:2004.9Imidazolium-type room temperature ionic liquids (RTILs) have been used for the Stetter reaction, affording the desired 1,4-dicarbonyl compounds in good yields. Thiazolium salts and Et3N are efficient catalysts for this reaction performed in ionic liquid. The possibility to recycle and reuse the solvent has been demonstrated, although it was not possible to recycle the thiazolium catalyst. This method
-
Catalytic Action of Triarylstibanes: Oxidation of Benzoins into Benzyls Using Triarylstibanes Under an Aerobic Condition作者:Shuji Yasuike、Yoshihito Kishi、Shin-ichiro Kawara、Jyoji KuritaDOI:10.1248/cpb.53.425日期:——Benzoins are simply oxidized to benzils in excellent yields with a catalytic amount of triarylstibanes under an aerobic condition. This catalytic oxidation is heteroatom-specific in the antimony compound and no reaction take place with other group 15 reagents such as triphenylphosphane, -arsane and -bismuthane. The reaction should involve an oxidation-reduction cycle between stibane Sb(III) and stiborane
表征谱图
-
氢谱1HNMR
-
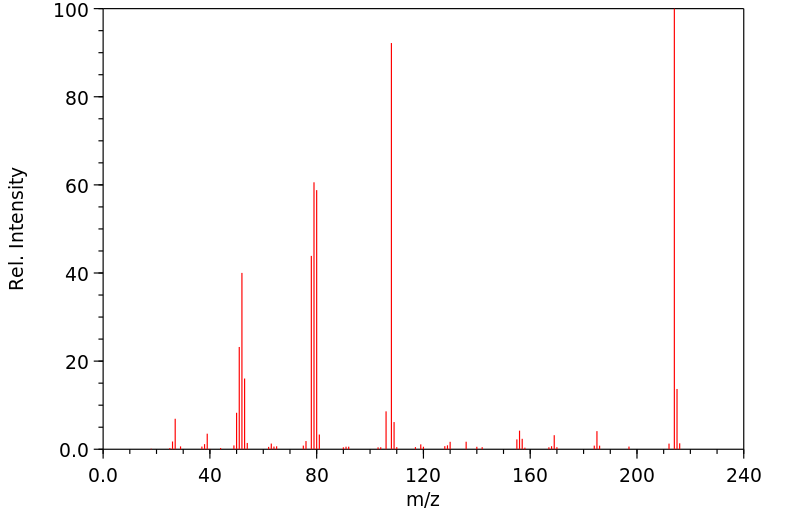
质谱MS
-
碳谱13CNMR
-
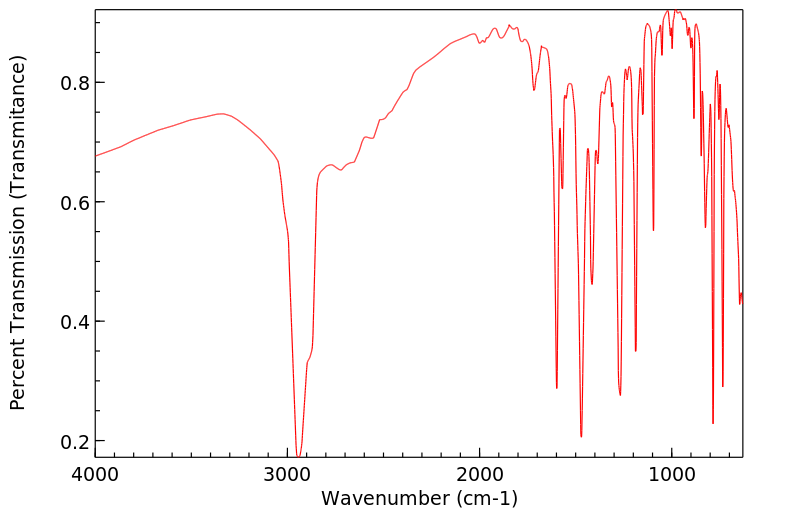
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷