尼泊金丁酯 | 94-26-8
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:67-70 °C(lit.)
-
沸点:156-157 °C3.5 mm Hg(lit.)
-
密度:1.28
-
闪点:181℃
-
溶解度:溶于DMSO、乙酸乙酯、甲醇。
-
LogP:3.57
-
物理描述:N-butyl-p-hydroxybenzoate appears as odorless white crystals or crystalline powder. Tasteless, but numbs the tongue. Aqueous solutions slightly acidic to litmus. (NTP, 1992)
-
颜色/状态:Small, colorless crystals or powder
-
气味:Odorless
-
蒸汽压力:2.51X10-4 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
自燃温度:495 °C
-
分解:When heated to decomposition it emits acrid smoke and irritating fumes.
-
解离常数:pKa = 8.47
-
碰撞截面:150.14 Ų [M-H]-
-
保留指数:1671 ;1665
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:14
-
可旋转键数:5
-
环数:1.0
-
sp3杂化的碳原子比例:0.363
-
拓扑面积:46.5
-
氢给体数:1
-
氢受体数:3
ADMET
安全信息
-
TSCA:Yes
-
安全说明:S22,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:2918290000
-
危险品运输编号:NONH for all modes of transport
-
RTECS号:DH1980000
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:密封、阴凉、干燥处保存。
SDS
模块 1. 化学品
1.1 产品标识符
: 4-羟基苯甲酸丁酯
产品名称
1.2 鉴别的其他方法
无数据资料
1.3 有关的确定了的物质或混合物的用途和建议不适合的用途
仅供科研用途,不作为药物、家庭备用药或其它用途。
模块 2. 危险性概述
2.1 GHS分类
皮肤刺激 (类别3)
2.2 GHS 标记要素,包括预防性的陈述
象形图 无
警示词 警告
危险申明
H316 造成轻微皮肤刺激。
警告申明
措施
P332 + P313 如发生皮肤刺激:求医/ 就诊。
2.3 其它危害物 - 无
模块 3. 成分/组成信息
3.1 物 质
: C11H14O3
分子式
: 194.23 g/mol
分子量
组分 浓度或浓度范围
Butylparaben
-
CAS 号 94-26-8
EC-编号 202-318-7
模块 4. 急救措施
4.1 必要的急救措施描述
一般的建议
请教医生。 出示此安全技术说明书给到现场的医生看。
吸入
如果吸入,请将患者移到新鲜空气处。 如果停止了呼吸,给于人工呼吸。 请教医生。
皮肤接触
用肥皂和大量的水冲洗。 请教医生。
眼睛接触
用水冲洗眼睛作为预防措施。
食入
切勿给失去知觉者从嘴里喂食任何东西。 用水漱口。 请教医生。
4.2 主要症状和影响,急性和迟发效应
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
4.3 及时的医疗处理和所需的特殊处理的说明和指示
无数据资料
模块 5. 消防措施
5.1 灭火介质
灭火方法及灭火剂
用水雾,耐醇泡沫,干粉或二氧化碳灭火。
5.2 源于此物质或混合物的特别的危害
碳氧化物
5.3 给消防员的建议
如必要的话,戴自给式呼吸器去救火。
5.4 进一步信息
无数据资料
模块 6. 泄露应急处理
6.1 人员的预防,防护设备和紧急处理程序
使用个人防护设备。 防止粉尘的生成。 防止吸入蒸汽、气雾或气体。 保证充分的通风。 避免吸入粉尘。
6.2 环境保护措施
不要让产物进入下水道。
6.3 抑制和清除溢出物的方法和材料
收集、处理泄漏物,不要产生灰尘。 扫掉和铲掉。 存放进适当的闭口容器中待处理。
6.4 参考其他部分
丢弃处理请参阅第13节。
模块 7. 操作处置与储存
7.1 安全操作的注意事项
在有粉尘生成的地方,提供合适的排风设备。
7.2 安全储存的条件,包括任何不兼容性
贮存在阴凉处。 容器保持紧闭,储存在干燥通风处。
7.3 特定用途
无数据资料
模块 8. 接触控制和个体防护
8.1 容许浓度
最高容许浓度
没有已知的国家规定的暴露极限。
8.2 暴露控制
适当的技术控制
按照良好工业和安全规范操作。 休息前和工作结束时洗手。
个体防护设备
眼/面保护
带有防护边罩的安全眼镜符合 EN166要求请使用经官方标准如NIOSH (美国) 或 EN 166(欧盟)
检测与批准的设备防护眼部。
皮肤保护
戴手套取 手套在使用前必须受检查。
请使用合适的方法脱除手套(不要接触手套外部表面),避免任何皮肤部位接触此产品.
使用后请将被污染过的手套根据相关法律法规和有效的实验室规章程序谨慎处理. 请清洗并吹干双手
所选择的保护手套必须符合EU的89/686/EEC规定和从它衍生出来的EN 376标准。
身体保护
根据危险物质的类型,浓度和量,以及特定的工作场所来选择人体保护措施。,
防护设备的类型必须根据特定工作场所中的危险物的浓度和含量来选择。
呼吸系统防护
不需要保护呼吸。如需防护粉尘损害,请使用N95型(US)或P1型(EN 143)防尘面具。
呼吸器使用经过测试并通过政府标准如NIOSH(US)或CEN(EU)的呼吸器和零件。
模块 9. 理化特性
9.1 基本的理化特性的信息
a) 外观与性状
形状: 粉末
颜色: 白色
b) 气味
无数据资料
c) 气味阈值
无数据资料
d) pH值
无数据资料
e) 熔点/凝固点
熔点/凝固点: 68 - 70 °C
熔点/凝固点: 67 - 70 °C - lit.
f) 起始沸点和沸程
156 - 157 °C 在 4.7 hPa - lit.
g) 闪点
无数据资料
h) 蒸发速率
无数据资料
i) 易燃性(固体,气体)
无数据资料
j) 高的/低的燃烧性或爆炸性限度 无数据资料
k) 蒸汽压
无数据资料
l) 蒸汽密度
无数据资料
m) 相对密度
无数据资料
n) 水溶性
无数据资料
o) n-辛醇/水分配系数
无数据资料
p) 自燃温度
无数据资料
q) 分解温度
无数据资料
r) 粘度
无数据资料
模块 10. 稳定性和反应活性
10.1 反应性
无数据资料
10.2 稳定性
无数据资料
10.3 危险反应的可能性
无数据资料
10.4 应避免的条件
无数据资料
10.5 不兼容的材料
强氧化剂
10.6 危险的分解产物
其它分解产物 - 无数据资料
模块 11. 毒理学资料
11.1 毒理学影响的信息
急性毒性
半数致死剂量 (LD50) 经口 - 老鼠 - 13,200 mg/kg
皮肤刺激或腐蚀
皮肤 - 豚鼠 - 轻度的皮肤刺激 - 48 h
眼睛刺激或腐蚀
无数据资料
呼吸道或皮肤过敏
无数据资料
生殖细胞突变性
无数据资料
致癌性
IARC:
此产品中没有大于或等于 0。1%含量的组分被 IARC鉴别为可能的或肯定的人类致癌物。
生殖毒性
无数据资料
特异性靶器官系统毒性(一次接触)
无数据资料
特异性靶器官系统毒性(反复接触)
无数据资料
吸入危险
无数据资料
潜在的健康影响
吸入 吸入可能有害。 可能引起呼吸道刺激。
摄入 如服入是有害的。
皮肤 如果通过皮肤吸收可能是有害的。 可能引起皮肤刺激。
眼睛 可能引起眼睛刺激。
接触后的征兆和症状
据我们所知,此化学,物理和毒性性质尚未经完整的研究。
附加说明
化学物质毒性作用登记: DH1980000
模块 12. 生态学资料
12.1 生态毒性
无数据资料
12.2 持久存留性和降解性
无数据资料
12.3 潜在的生物蓄积性
无数据资料
12.4 土壤中的迁移性
无数据资料
12.5 PBT 和 vPvB的结果评价
无数据资料
12.6 其它不利的影响
无数据资料
模块 13. 废弃处置
13.1 废物处理方法
产品
将剩余的和未回收的溶液交给处理公司。
与易燃溶剂相溶或者相混合,在备有燃烧后处理和洗刷作用的化学焚化炉中燃烧
受污染的容器和包装
作为未用过的产品弃置。
模块 14. 运输信息
14.1 联合国危险货物编号
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.2 联合国(UN)规定的名称
欧洲陆运危规: 非危险货物
国际海运危规: 非危险货物
国际空运危规: 非危险货物
14.3 运输危险类别
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.4 包裹组
欧洲陆运危规: - 国际海运危规: - 国际空运危规: -
14.5 环境危险
欧洲陆运危规: 否 国际海运危规 海运污染物: 否 国际空运危规: 否
14.6 对使用者的特别提醒
无数据资料
模块 15 - 法规信息
N/A
模块16 - 其他信息
N/A
制备方法与用途
尼泊金丁酯是一种白色结晶性粉末,无臭、无味,易溶于乙醇。作为防腐剂时,常与异丙酯、丁酯等混合使用。
应用尼泊金丁酯具有广谱抑菌作用,能够有效抑制革兰氏阳性、阴性菌以及酵母菌和霉菌的生长。
含量分析取试样2g(准确至0.1mg),在硅胶上预先干燥5h,移入烧瓶。加1mol/L氢氧化钠40.0ml,并用水冲洗烧瓶侧壁。盖上表面皿,在小火下煮沸1小时后冷却。加入溴百里酚蓝试液(TS-56)5滴,用1mol/L硫酸滴定过量的氢氧化钠,使溶液颜色与含相同比例指示剂的缓冲液(pH 6.5)一致。另作试剂空白试验,并进行必要的校正。每毫升1mol/L氢氧化钠相当于本品(C11H14O3)194.2mg。
毒性尼泊金丁酯的ADI值延期决定(FAO/WHO,2001)。LD50值为16.0g/kg(小鼠,皮下注射)。该物质在小鼠短期毒性试验中有抑制体重增加、引起急性皮炎等报告。在对羟基苯甲酸酯类中,尼泊金丁酯的防腐效果最佳,但其毒性也较大。
使用限量日本(1998年,以对羟基苯甲酸计,括号内数据为折合成本品的量,g/kg):酱油0.25g/L(0.35g/L)、食醋0.1g/L(0.14g/L);清凉饮料及糖浆0.1(0.14);水果调味酱0.2(0.28);果蔬0.012(0.016)。
化学性质尼泊金丁酯为白色结晶粉末,稍有特殊臭味。微溶于水,易溶于醇、醚和三氯甲烷。
用途用于药品、化妆品及食品的抑菌和防腐。作为防腐剂时,常配制成乙醇溶液、乙酸溶液或氢氧化钠溶液,并常用几种不同的酯类混合使用以提高溶解度。例如,三份异丁酯、四份异丙酯、三份丁酯配制成共溶混合物,此混合物可作为水包油型乳化剂用于酱油等食品中,其溶解度比单用丁酯可提高2~3倍。应用于酱油时,可在5%的氢氧化钠溶液中添加本品20%~25%,再缓慢加入已加热至约80℃的酱油内;亦可使用醋酸溶液或酯类乳化剂。对酱油防霉的用量为0.05~0.10g/L。在食醋中可利用醋酸或氢氧化钠溶液,通常与苯甲酸及脱氢醋酸合用。此外,尼泊金丁酯还可用作有机合成中间体、医药、食品、化妆品、胶片及高档产品的防腐添加剂和试剂。
生产方法由对羟基苯甲酸与丁醇酯化而得。将丁醇、对羟基苯甲酸一起加热溶解,慢慢滴加硫酸,反应8小时后冷却,加入4%碳酸钠溶液中分出水层,通蒸气赶出丁醇,放冷过滤得粗品,再用乙醇重结晶(在乙醇中的溶解度:200g/100ml)。
生产方法 类别有毒物质
毒性分级中毒
急性毒性口服-小鼠 LD50: 13200 毫克/公斤;腹腔-小鼠 LD50: 230 毫克/公斤
刺激数据皮肤-豚鼠 5%/48小时 轻度
可燃性危险特性热分解,辛辣刺激烟雾
储运特性库房通风、低温干燥
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 4-(甲氧基甲氧基)苯甲酸正丁酯 n-butyl 4-(methoxymethoxy)benzoate 251640-53-6 C13H18O4 238.284 对羟基苯甲酸甲酯 methyl 4-hydroxybenzoate 99-76-3 C8H8O3 152.15 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(甲氧基甲氧基)苯甲酸正丁酯 n-butyl 4-(methoxymethoxy)benzoate 251640-53-6 C13H18O4 238.284 —— n-butyl 4-n-butoxybenzoate 4906-27-8 C15H22O3 250.338 —— 4-Acetoxybenzoic acid butyl ester 27739-14-6 C13H16O4 236.268 —— butyl 4-trimethylsiloxybenzoate —— C14H22O3Si 266.412 —— butyl-4-benzyloxybenzoate 56442-26-3 C18H20O3 284.355 —— 4-methallyloxy-benzoic acid butyl ester —— C15H20O3 248.322 —— 4-carbamoyloxy-benzoic acid butyl ester —— C12H15NO4 237.255 4-甲基-苯甲酸丁酯 n-butyl 4-methylbenzoate 19277-56-6 C12H16O2 192.258 4-乙酰氧基苯甲酸丙酯 n-propyl 4-acetoxybenzoate 27739-13-5 C12H14O4 222.241 —— 4-(2,3-dihydroxy-propoxy)-benzoic acid butyl ester —— C14H20O5 268.31 —— 2-Methylpropyl 4-acetyloxybenzoate 27801-52-1 C13H16O4 236.268 —— butyl 4-[(aminosulphonyl)oxy]benzoate —— C11H15NO5S 273.31 —— DCW234 723759-26-0 C15H20O4 264.321 乙基4-乙酰氧基苯甲酸酯 ethyl 4-acetoxybenzoate 13031-45-3 C11H12O4 208.214 —— butyl 3-bromo-4-hydroxybenzoate 37470-61-4 C11H13BrO3 273.126 —— 4-dimethylcarbamoyloxy-benzoic acid butyl ester —— C14H19NO4 265.309 —— Isopropyl 4-acetoxybenzoate 27739-15-7 C12H14O4 222.241 4-羟基-3,5-二碘苯甲酸丁酯 butyl 3,5-di-iodo-4-hydroxybenzoate 51-38-7 C11H12I2O3 446.023 —— butyl 3,5-di-chloro-4-hydroxybenzoate —— C11H12Cl2O3 263.12 —— di(p-n-butyloxycarbonylphenyl)oxalate 105653-10-9 C24H26O8 442.466 —— Di(p-butoxycarbonylphenyl)terephthalate —— C30H30O8 518.6 对羟基苯甲酸 4-hydroxy-benzoic acid 99-96-7 C7H6O3 138.123 —— butyl 3,5-di-bromo-4-hydroxybenzoate 75254-69-2 C11H12Br2O3 352.022 —— n-butyl p-(p-n-butyloxybenzoyloxy)benzoate 155555-55-8 C22H26O5 370.445 4-乙酰氧基苯甲酸甲酯 methyl 4-acetoxybenzoate 24262-66-6 C10H10O4 194.187 —— butyl p-[p'-n-octyloxybenzoyloxy]benzoate —— C26H34O5 426.553 —— butyl [1,1′-biphenyl]-4-carboxylate —— C17H18O2 254.329 —— butyl 4-(((trifluoromethyl)sulfonyl)oxy)benzoate 1195931-62-4 C12H13F3O5S 326.293 —— Benzyl-p-acetoxy-benzoat 54835-09-5 C16H14O4 270.285 —— 4-phenylcarbamoyloxy-benzoic acid butyl ester —— C18H19NO4 313.353 —— 4-(piperidine-1-carbonyloxy)-benzoic acid butyl ester —— C17H23NO4 305.374 4-(甲氧基甲基)苯甲酸 4-(methoxymethoxy)benzoic acid 25458-44-0 C9H10O4 182.176 —— 4-(4-methoxy-phenylcarbamoyloxy)-benzoic acid butyl ester —— C19H21NO5 343.379 —— (4-butoxycarbonyl-phenyl)-carbamic acid-(4-butoxycarbonyl-phenyl ester) —— C23H27NO6 413.47 —— 3,3'-thiobis(butyl p-hydroxybenzoate) 107490-71-1 C22H26O6S 418.511 —— 4-(4-chloro-phenylcarbamoyloxy)-benzoic acid butyl ester —— C18H18ClNO4 347.798 - 1
- 2
- 3
- 4
反应信息
-
作为反应物:参考文献:名称:Alkyl trimethylsiloxybenzoat compounds and method of preparation摘要:该发明提供了通式R'3SiO-C6H4-COOR的高纯度流体烷基三有机硅氧基苯甲酸酯,其中R'是一价的具有1至约6个碳原子的脂肪烃取代基或一价的芳香烃取代基,-C6H4-是二取代芳香环,R是一价的具有1至约20个碳原子的脂肪烃取代基,其中这些烷基三有机硅氧基苯甲酸酯是通过一种新颖的方法制备的,并且可以轻松地与含有有机成分的化妆品配方一起加热,并在所述化妆品配方中提供与它们的前体固体烷基羟基苯甲酸酯提供的防腐性能相媲美的防腐性能。公开号:EP1205482A3
-
作为产物:描述:参考文献:名称:An Efficient and Selective Deprotecting Method for Methoxymethyl Ethers摘要:Methoxymethyl ethers were selectively deprotected to the corresponding phenols in high yields by CBr4 and PPh3 in aprotic solvent (CICH2CH2Cl) under slightly thermal reaction conditions.DOI:10.1081/scc-200039382
文献信息
-
[EN] ANTIBACTERIAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIBACTÉRIENS申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2019199979A1公开(公告)日:2019-10-17The present application provides compounds of formula: Methods of using these compounds for killing bacterial growth and treating bacterial infections are also provided.本申请提供了以下化合物的公式:还提供了使用这些化合物杀灭细菌生长和治疗细菌感染的方法。
-
[EN] PRMT5 INHIBITORS CONTAINING A DIHYDRO- OR TETRAHYDROISOQUINOLINE AND USES THEREOF<br/>[FR] INHIBITEURS DE LA PRMT5 CONTENANT UNE DIHYDRO- OU TÉTRAHYDRO-ISOQUINOLÉINE ET LEURS UTILISATIONS申请人:EPIZYME INC公开号:WO2014100730A1公开(公告)日:2014-06-26Described herein are compounds of Formula (A), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5- mediated disorders are also described.
-
PRMT5 INHIBITORS AND USES THEREOF申请人:Duncan Kenneth W.公开号:US20190083482A1公开(公告)日:2019-03-21Described herein are compounds of Formula (I), pharmaceutically acceptable salts thereof, and pharmaceutical compositions thereof. Compounds of the present invention are useful for inhibiting PRMT5 activity. Methods of using the compounds for treating PRMT5-mediated disorders are also described.
-
Zr‐MOF‐808 as Catalyst for Amide Esterification作者:Beatriz Villoria‐del‐Álamo、Sergio Rojas‐Buzo、Pilar García‐García、Avelino CormaDOI:10.1002/chem.202003752日期:2021.3.8esterification. Comparing with previously reported homogeneous and heterogeneous catalysts, Zr‐MOF‐808‐P can promote the reaction for a wide range of primary, secondary and tertiary amides with n‐butanol as nucleophilic agent. Different alcohols have been employed in amide esterification with quantitative yields. Moreover, the catalyst acts as a heterogeneous catalyst and could be reused for at least five
-
[EN] NOVEL COMPOUNDS<br/>[FR] NOUVEAUX COMPOSÉS申请人:UCL BUSINESS LTD公开号:WO2019243841A1公开(公告)日:2019-12-26The present invention relates to compounds, compositions, combinations and medicaments containing said compounds and processes for their preparation. The invention also relates to the use of said compounds, combinations, compositions and medicaments, for example as modulators of alpha 1 antitrypsin and treating diseases associated with alpha antitrypsin, particularly liver diseases.
表征谱图
-
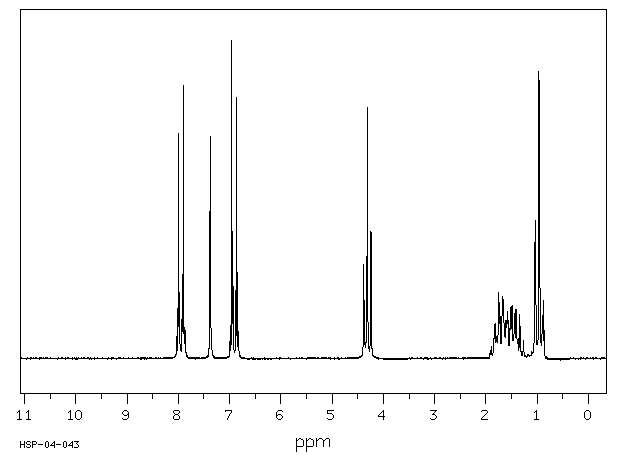
氢谱1HNMR
-
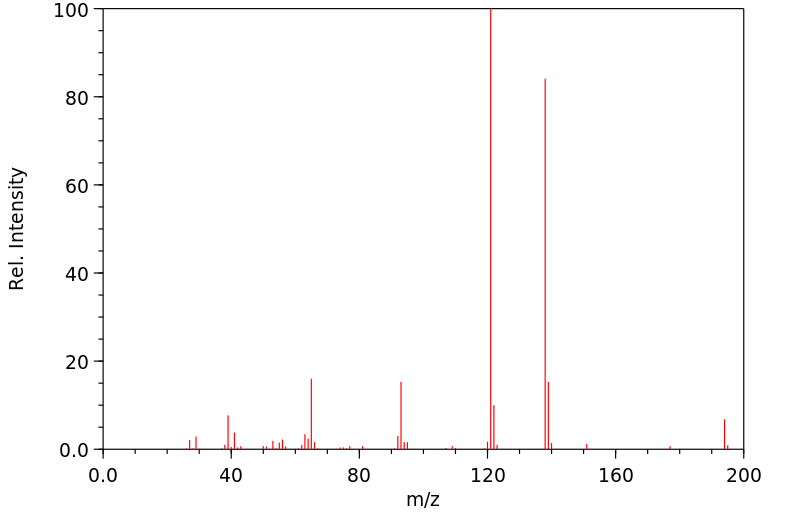
质谱MS
-
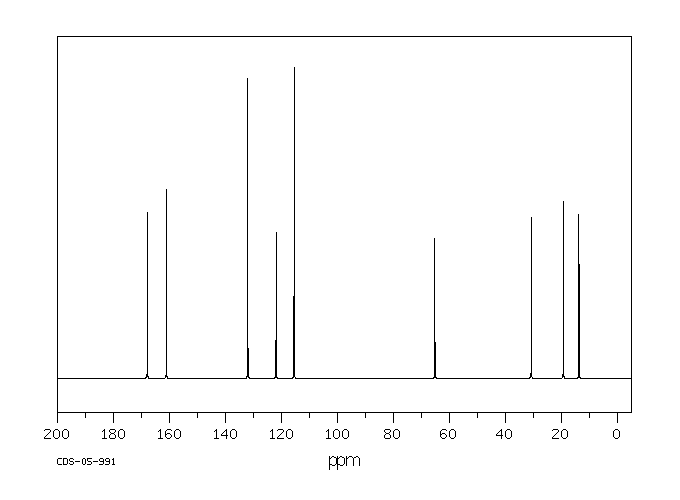
碳谱13CNMR
-
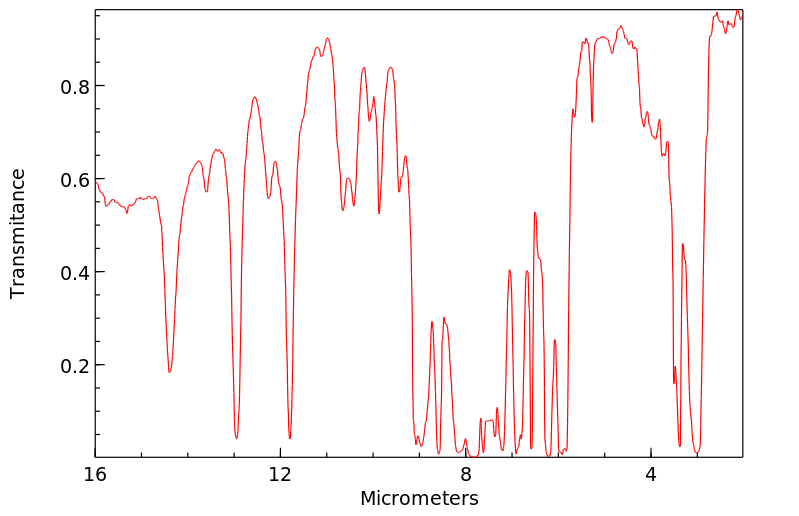
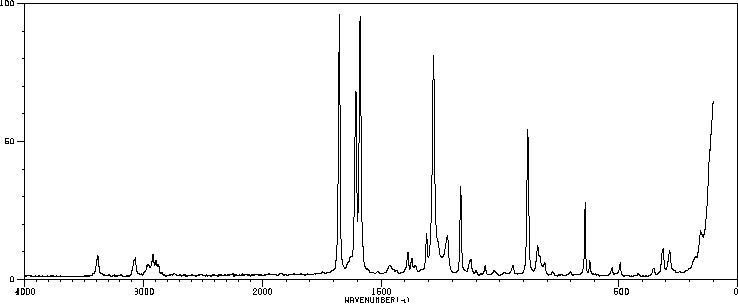
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息