异丙基乙烯基醚 | 926-65-8
物质功能分类
中文名称
异丙基乙烯基醚
中文别名
乙烯基异丙醚;乙烯基异丙基醚
英文名称
vinyl isopropyl ether
英文别名
iso-propyl vinyl ether;2-(vinyloxy)propane;Propane, 2-(ethenyloxy)-;2-ethenoxypropane
CAS
926-65-8
化学式
C5H10O
mdl
MFCD00053718
分子量
86.1338
InChiKey
GNUGVECARVKIPH-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-140°C
-
沸点:68.84°C (estimate)
-
密度:0.7534
-
稳定性/保质期:
在常温常压下保持稳定,应避免与强氧化剂、强酸及氧气直接接触。
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:6
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.6
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
危险等级:3.1
-
海关编码:2909199090
-
包装等级:II
-
危险类别:3.1
-
危险品运输编号:3271
-
储存条件:请将容器密封保存,并存放在阴凉、干燥处。
SDS
反应信息
-
作为反应物:参考文献:名称:Schostakowskii et al., Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1954, p. 303,305,311;engl.Ausg.S.245,247,250,252摘要:DOI:
-
作为产物:描述:参考文献:名称:Woronkow, Zhurnal Obshchei Khimii, 1950, vol. 20, p. 2061摘要:DOI:
文献信息
-
METHOD FOR PRODUCING PYRAZOLE FUSED RING DERIVATIVE申请人:Eisai R&D Management Co., Ltd.公开号:EP2202233A1公开(公告)日:2010-06-30Disclosed is a commercially advantageous method for producing a pyrazole fused ring derivative (such as a 7-phenylpyrazolo[1,5-a]pyridine derivative). Specifically disclosed is a method for producing a compound (I) represented by the formula (I) below or a salt thereof, which comprises a step A wherein a hydroxy group in a compound (IV) represented by the formula (IV) below is converted into a methoxy group, thereby obtaining a compound (I) or a salt thereof:
-
Visible-Light-Driven Nitrogen Radical-Catalyzed [3 + 2] Cyclization of Vinylcyclopropanes and <i>N</i>-Tosyl Vinylaziridines with Alkenes作者:Quan-Qing Zhao、Xue-Song Zhou、Shuang-Hua Xu、Ya-Li Wu、Wen-Jing Xiao、Jia-Rong ChenDOI:10.1021/acs.orglett.0c00712日期:2020.3.20photoredox-promoted and nitrogen radical catalyzed [3 + 2] cyclization of vinylcyclopropanes and N-tosyl vinylaziridines with alkenes is developed. Key to the success of this process is the use of the readily tunable hydrazone as a nitrogen radical catalyst. Preliminary mechanism studies suggest that the photogenerated nitrogen radical undergoes reversible radical addition to the vinylcyclopropanes
-
[4++2]-Type polar cycloadditions of 2-benzothiopyrylium salt with alkenes作者:Hiroshi Shimizu、Naoko Araki、Osamu Muraoka、Genzoh TanabeDOI:10.1016/s0040-4039(00)00124-6日期:2000.3Treatment of 2-benzothiopyrylium salt with alkenes such as styrene, p-methylstyrene, p-methoxystyrene, α-methylstyrene, and trans-anethole afforded the corresponding [4++2]-type polar cycloaddition products, respectively. The structures of the cycloadducts were confirmed by X-ray crystal structure determination of the corresponding sulfone derivative.
-
Visible Light-Induced Photoredox Construction of Trifluoromethylated Quaternary Carbon Centers from Trifluoromethylated Tertiary Bromides作者:Feng Huan、Qing-Yun Chen、Yong GuoDOI:10.1021/acs.joc.6b00930日期:2016.8.19prolonged reaction times. For aliphatic alkenes, the reactions were neither thermodynamic nor kinetic and fac-Ir(ppy)3 was used as catalyst. Thus, reactions were not as efficient as electron-rich alkenes. The atom-transfer radical addition reactions of trifluoromethylated tertiary bromides with alkynes were also achieved. The configuration of products we separated was E type only. Some of the products exhibited已经开发了一种温和的,操作简单的,可见光诱导的光氧化还原方法,用于从三氟甲基化的叔溴化物中构建新型的三氟甲基化的季碳中心。使用这种方法,多种烯烃成功地双官能化为γ-丁内酰胺。对于富电子烯烃,Ir(dF(CF 3)ppy)2(dtbbpy)(PF 6)催化的反应是动力学过程,产率高,反应时间短。对于苯乙烯,由Ir(ppy)2(dtbbpy)(PF 6)催化的反应是热力学过程,具有中等收率和延长的反应时间。对于脂族烯烃,反应既不是热力学也不是动力学,反应是fac -Ir(ppy)3用作催化剂。因此,反应不如富电子烯烃有效。还实现了三氟甲基化叔溴化物与炔烃的原子转移自由基加成反应。我们分离的产品配置仅为E型。一些产品表现出杀菌活性。
-
Iron-Catalyzed Regioselective Anti-Markovnikov Addition of C–H Bonds in Aromatic Ketones to Alkenes作者:Naoki Kimura、Takuya Kochi、Fumitoshi KakiuchiDOI:10.1021/jacs.7b08385日期:2017.10.25alkylation reaction, in which the coupling of aromatic ketones with alkenes proceeds in the presence of only a simple Fe(PMe3)4 catalyst. The anti-Markovnikov addition of ortho C-H bonds in various ketones occurs with excellent regioselectivity under relatively mild reaction conditions. A strikingly wide variety of alkenes can be used for this reaction, and the high-yielding anti-Markovnikov addition我们在此报告了 CH 烷基化反应,其中芳香酮与烯烃的偶联仅在简单的 Fe(PMe3)4 催化剂存在下进行。在相对温和的反应条件下,各种酮中邻位 CH 键的反马尔可夫尼科夫加成具有优异的区域选择性。非常广泛的烯烃可用于该反应,并且使用该催化剂体系首次实现了芳族 CH 键与烯醇醚的高产反马尔科夫尼科夫加成。
表征谱图
-
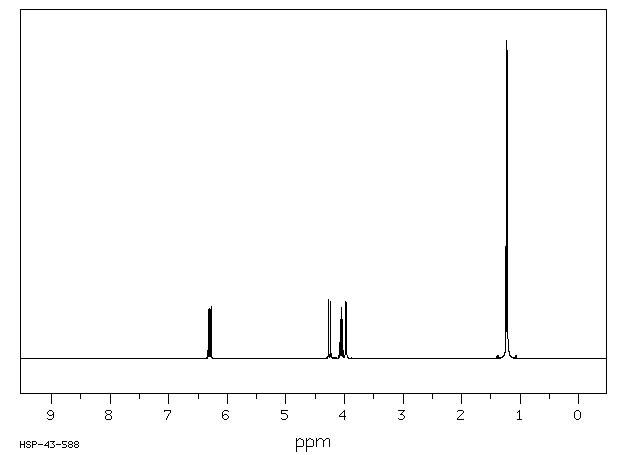
氢谱1HNMR
-
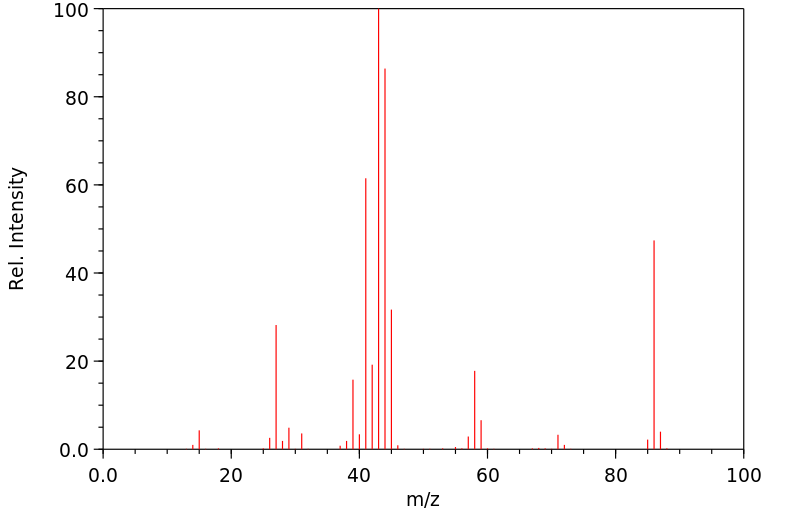
质谱MS
-
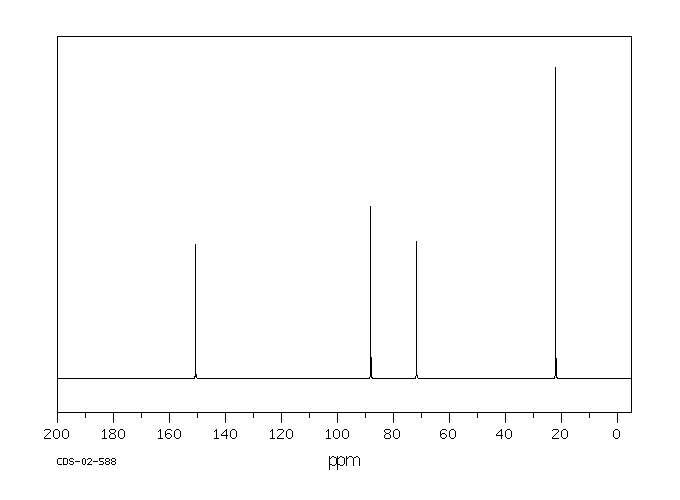
碳谱13CNMR
-
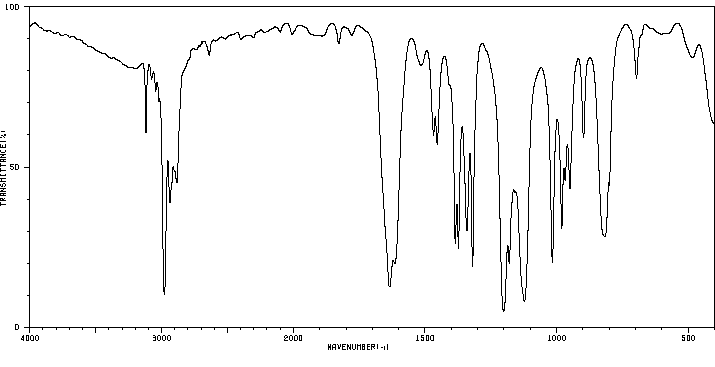
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷