1,3,5-环己三羧酸 | 25357-95-3
物质功能分类
中文名称
1,3,5-环己三羧酸
中文别名
顺,顺-1,3,5-环己三羧酸;(1Α,3Α,5Α)-1,3,5-环己三羧酸;(1α,3α,5α)-1,3,5-环己三羧酸;1,3,5-环己烷三羧酸;1,3,5-环己三酸;1,3,5-环己三羧酸(顺反异构体混合物)
英文名称
1,3,5-cyclohexanetricarboxylic acid
英文别名
cyclohexane-1,3,5-tricarboxylic acid;cis,cis-1,3,5-cyclohexanetricarboxylic acid;cis-1,3,5-cyclohexanetricarboxylic acid
CAS
25357-95-3
化学式
C9H12O6
mdl
MFCD23100819
分子量
216.191
InChiKey
FTHDNRBKSLBLDA-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:207 °C
-
沸点:372.4±42.0 °C(Predicted)
-
密度:1.509±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:15
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:112
-
氢给体数:3
-
氢受体数:6
安全信息
-
储存条件:室温且干燥
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,3,5-环己烷三甲酸三甲酯(顺反混合物) trimethyl cyclohexane-1,3,5-tricarboxylate 52831-11-5 C12H18O6 258.271
反应信息
-
作为反应物:描述:参考文献:名称:A novel reduction of polycarboxylic acids into their corresponding alkanes using n-butylsilane or diethylsilane as the reducing agent摘要:A convenient one-pot reaction has been developed for the reduction of polycarboxylic acids on aliphatic and aromatic systems to their corresponding alkanes. The reduction utilises either diethylsilane or n-butylsilane as the reducing agent in the presence of the Lewis acid catalyst tris(pentafluorophenyl)borane. (c) 2006 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2006.03.111
-
作为产物:描述:参考文献:名称:JP6083270摘要:公开号:
文献信息
-
POLYMER-CARBOHYDRATE CONJUGATES FOR DRUG DELIVERY TECHNOLOGY申请人:Wu Nian公开号:US20150157721A1公开(公告)日:2015-06-11The invention comprises compounds, methods of making, and methods of using. The compounds may have a linear or cylic backbone and three or four appended functional groups: one or two lipohilic compounds including sterols or “fat soluble” vitamins, one or two hydrophilic polymer, and one or two carbohydrate. A group of polymer-carbohydrate conjugates having a central backbone and three appended functional groups are disclosed wherein one lipophilic compound is void of both steroid acids. The conjugate may have fatty acids as the primary lipophilic carrier, one hydrophilic polymer, and one carbohydrate. Specific functional groups may be selected for specific applications in formulating pharmaceuticals, cosmetics, nutriceuticals, and the like. Typical coupling reaction of the conjugates may involve one or more or combinations or in series of alkylation including N-alkylation or O-alkylation, etherification, esterification and amidation chemical processes. A variety of linkers between the backbone and functional groups may also be selected to modify the carriers or center backbones for the coupling reactions and optimize performance of the conjugates.该发明包括化合物、制备方法和使用方法。这些化合物可能具有线性或环状的骨架,以及三个或四个附加的功能基团:一个或两个疏水化合物,包括固醇或“脂溶性”维生素,一个或两个亲水性聚合物,以及一个或两个碳水化合物。公开了一组具有中心骨架和三个附加功能基团的聚合物-碳水化合物共轭物,其中一个疏水性化合物不含类固醇酸。该共轭物可能以脂肪酸作为主要疏水载体,一个亲水性聚合物和一个碳水化合物。特定的功能基团可以根据在制备药物、化妆品、营养保健品等方面的具体应用而选择。共轭物的典型偶联反应可能涉及一种或多种或组合或串联的烷基化,包括N-烷基化或O-烷基化,醚化,酯化和酰胺化化学过程。还可以选择各种连接剂连接骨架和功能基团之间,以修改载体或中心骨架以进行偶联反应并优化共轭物的性能。
-
LIGHT-DRIVEN ROTARY MOLECULAR MOTORS申请人:Bell Thomas W.公开号:US20110077394A1公开(公告)日:2011-03-31Compounds of Formula (1) are disclosed. C b is a carbocyclic or heterocyclic group having an atom within the cyclic structure selected from C, N, Si, and C r and singly bound to A. A is CR, COR, CSR, CNR 2 , CCN, CCONR 2 , CNO 2 , CNNAr, CX′, or N. C r is a chromophore having a substantially planar cyclic structure. The compounds function as nanometer-scale rotary molecular motors powered and controlled by light energy. The design of the molecular motor devices is flexible so that the rotary direction, drive light wavelength, and other physical characteristics can be varied. The compounds can be chemically functionalized to allow it to be integrated into or attached to a variety of structures. The device can be used in applications where mechanical power, positional control, and information encoding are to be generated at the size scale of individual molecules.式(1)的化合物已被披露。 C b 是一个具有环结构内选自C、N、Si和C r 的原子的碳环或杂环基团,并与A单键相连。A是CR、COR、CSR、CNR 2 、CCN、CCONR 2 、CNO 2 、CNNAr、CX′或N。C r 是具有基本平面环结构的色团。这些化合物作为受光能驱动和控制的纳米尺度旋转分子马达。分子马达装置的设计灵活,使得旋转方向、驱动光波长和其他物理特性可以变化。这些化合物可以在化学上官能化,以使其能够集成到或附着到各种结构中。该装置可用于需要在单个分子尺度上产生机械动力、位置控制和信息编码的应用中。
-
[EN] POLYMER-CYCLODEXTRIN-LIPID CONJUGATES<br/>[FR] CONJUGUÉS POLYMÈRE-CYCLODEXTRINE-LIPIDE申请人:WU NIAN公开号:WO2016209732A1公开(公告)日:2016-12-29The invention comprises compounds, methods of making, and methods of using. A group of polymer-cyclodextrin-lipid conjugates having a center backbone and three or four appended functional groups are disclosed, wherein one of the hydrophilic components is cyclodextrin. The compounds may have a backbone with three or four appended functional groups: one or two lipophilic compounds including sterols or "fat soluble" vitamins or fatty acids, one or two hydrophilic polymer and one cyclodextrin. Specific functional groups may be selected for specific applications in formulating pharmaceuticals, cosmetics, nutriceuticals, and the like. Typical coupling reaction of the conjugates may involve one or more or combinations or in series of alkylation including N-alkylation or O-alkylation, etherification, esterification and amidation chemical processes. A variety of linkers between the center backbone and functional groups may also be selected to modify the carriers or center backbones for the coupling reactions and optimize performance of the conjugates.
-
Tuning the Supramolecular Structure through Variation of the Ligand Geometry and Metal Substituents–Diorganotin Macrocycles and Coordination Polymers Derived from <i>cis</i>- and <i>trans</i>-1,2-, 1,3-, and 1,4-Cyclohexanedicarboxylic and <i>cis</i>,<i>cis</i>-1,3,5-Cyclohexanetricarboxylic Acid作者:Irán Fernando Hernández-Ahuactzi、Jorge Cruz-Huerta、Hugo Tlahuext、Victor Barba、Jorge Guerrero-Alvarez、Herbert HöpflDOI:10.1021/cg501629n日期:2015.2.4to determine whether macrocyclic or polymeric diorganotin dicarboxylates are formed dependent of the spatial orientation of the coordinating ligand functions and the organic substituents at the metal atom and to analyze conformational and topological variations in the resulting supramolecular aggregates. The single-crystal X-ray diffraction studies showed that besides the ligand geometry the substituents顺式-和反式-1,2- chdcaH 2,1,3- chdcaH 2,和1,4- chdcaH 2(chdcaH 2 =环己烷二羧酸)以及顺式,顺式-1,3,5- chtcaH 3(chtcaH 3 =环己烷三羧酸)已用二甲基和二正丁基锡试剂处理过,对于1,4-chdcaH 2,还用二叔丁基试剂处理过。-丁基二氯化锡,以确定是否形成大环或聚合二有机锡二羧酸盐,具体取决于配位体官能团和金属原子上有机取代基的空间取向,并分析所得超分子聚集体的构象和拓扑变化。单晶X射线衍射研究表明,除了配体的几何形状以外,金属中心的取代基是形成单体,环寡聚或聚合物组装体的关键元素。其特征在于,X射线衍射分析中的化合物的两个表现出大环结构,[我2 SN(顺式- ë,一个-1,4- chdca)} 2 ]和[ Ñ卜2 SN(顺式- ë,一个-1,4- chdca)} 4 ]。对于大多数其余化合物,其组成为[R
-
AZIRIDINATED TRIGLYCERIDES AND POLYMERS FORMED THEREFROM申请人:Iowa State University Research Foundation, Inc.公开号:US20160289218A1公开(公告)日:2016-10-06Various embodiments disclosed relate to aziridinated triglycerides and polymers formed therefrom. In various embodiments, the present invention provides an aziridinated triglyceride having the structure described herein. In various embodiments, the present invention provides a method of making the aziridinated triglyceride, a method of crosslinking the aziridinated triglyceride to form a polymer, the polymer formed from the aziridinated triglyceride, a triglyceride with at least one pendent unsaturated group that can be used to form the aziridinated triglyceride, and a method of making the triglyceride having at least one pendent unsaturated group.
表征谱图
-
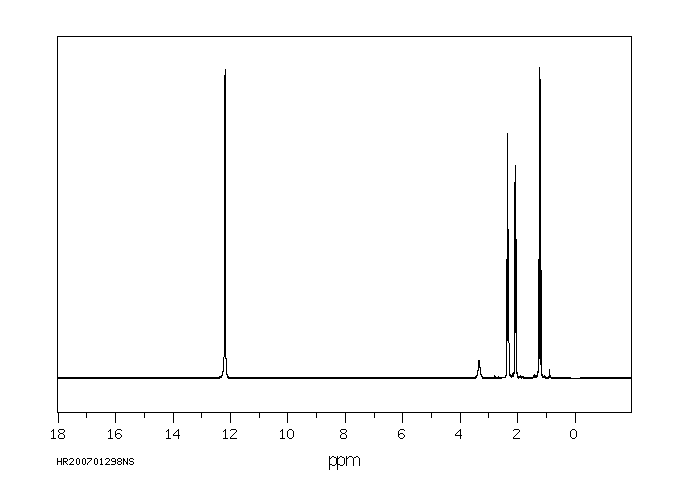
氢谱1HNMR
-
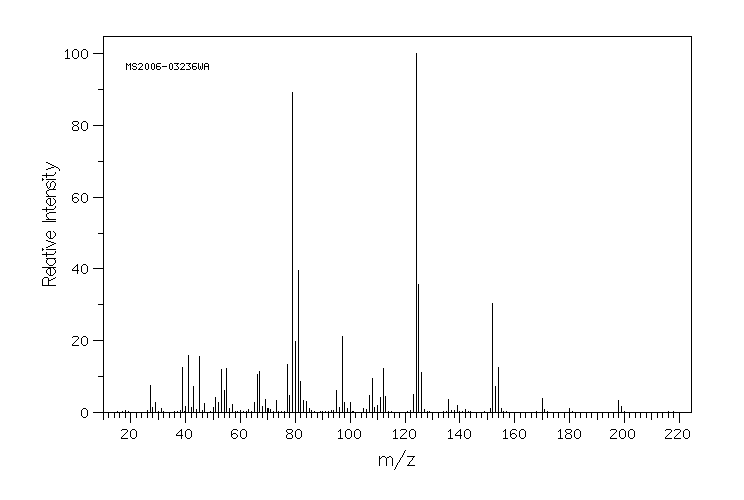
质谱MS
-
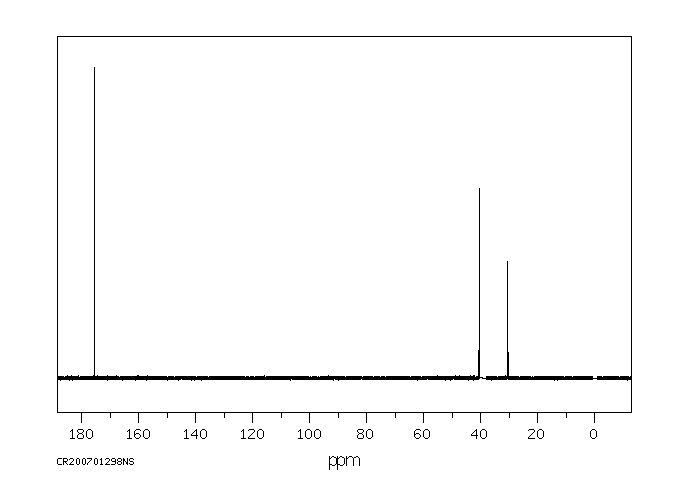
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸