1-(3-羟丙基)-2-吡咯烷酮 | 62012-15-1
中文名称
1-(3-羟丙基)-2-吡咯烷酮
中文别名
N-羟丙基-2-吡咯烷酮
英文名称
1-(3-hydroxypropyl)pyrrolidin-2-one
英文别名
N-(3-hydroxypropyl)-2-pyrrolidone;1-(3-hydroxypropyl)-2-pyrrolidone;1-(3-hydroxypropyl)-2-pyrrolidinone
CAS
62012-15-1
化学式
C7H13NO2
mdl
——
分子量
143.186
InChiKey
CVDGNRZPDAXOQO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:140 °C / 1mmHg
-
密度:1,1 g/cm3
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:10
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:0.86
-
拓扑面积:40.5
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xi
-
安全说明:S26,S36,S37,S39
-
危险类别码:R36/37/38
-
海关编码:2933790090
-
危险性防范说明:P264,P302+P352,P304+P340,P305+P351+P338,P332+P313,P337+P313
-
危险性描述:H315,H319,H335
-
储存条件:室温
SDS
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块 1. 化学品
产品名称: 1-(3-Hydroxypropyl)-2-pyrrolidone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-(3-羟丙基)-2-吡咯烷酮
百分比: >96.0%(GC)
CAS编码: 62012-15-1
分子式: C7H13NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 140 °C/0.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.11
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1-(3-Hydroxypropyl)-2-pyrrolidone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-(3-羟丙基)-2-吡咯烷酮
百分比: >96.0%(GC)
CAS编码: 62012-15-1
分子式: C7H13NO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
不适用的灭火剂: 棒状水
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。确保足够通风。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 用合适的吸收剂(如:旧布,干砂,土,锯屑)吸收泄漏物。一旦大量泄漏,筑堤控
制。附着物或收集物应该立即根据合适的法律法规废弃处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止烟雾产生。处理后彻底清洗双手
和脸。
注意事项: 如果蒸气或浮质产生,使用通风、局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防毒面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
液体
外形(20°C):
外观: 透明
颜色: 浅黄色-黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 140 °C/0.1kPa
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 1.11
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在装有后燃和洗涤装置的化学焚烧炉中焚烧。废弃处置时请遵守
国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-(3-羟丙基)-2-吡咯烷酮 修改号码:5
模块16 - 其他信息
N/A
制备方法与用途
(1-三羟甲基-2-吡咯烷酮)可以作为有机合成中间体和医药中间体,广泛应用于实验室研发和化工生产过程。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— ethyl 3-(2-oxopyrrolidin-1-yl)propanoate 61930-87-8 C9H15NO3 185.223 —— 1-[3-[(Tetrahydro-2H-pyran-2-yl)oxy]propyl]-2-pyrrolidinone 76243-34-0 C12H21NO3 227.304 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-(3-acetoxypropyl)pyrrolidin-2-one 62012-14-0 C9H15NO3 185.223 1-(3-氯丙基)-2-吡咯烷酮 1-(3-chloropropyl)pyrrolidin-2-one 91152-30-6 C7H12ClNO 161.631 —— 4-(2-oxopyrrolidin-1-yl)butanenitrile 98489-55-5 C8H12N2O 152.196 —— N-(3-acryloyloxypropyl)pyrrolidone 1220909-82-9 C10H15NO3 197.234 —— 1-[3-[(methylsulfonyl)oxy]propyl]-2-pyrrolidinone 1083430-07-2 C8H15NO4S 221.277 —— N-(3-Methacryloyloxy-propyl)-pyrrolidinon 76741-97-4 C11H17NO3 211.261
反应信息
-
作为反应物:描述:1-(3-羟丙基)-2-吡咯烷酮 在 氯化亚砜 、 sodium hydroxide 作用下, 以 水 为溶剂, 10.0~65.0 ℃ 、12.5 kPa 条件下, 反应 2.16h, 以65%的产率得到1-(3-氯丙基)-2-吡咯烷酮参考文献:名称:[EN] HYDROFORMYLATION PROCESS FOR THE CONVERSION OF AN ETHYLENICALLY UNSATURATED COMPOUND TO AN ALCOHOL
[FR] PROCESSUS D'HYDROFORMYLATION POUR LA CONVERSION D'UN COMPOSE INSATURE SUR LE PLAN ETHYLENIQUE EN UN ALCOOL摘要:本发明涉及一种水合甲酰化过程,用于将乙烯基不饱和化合物转化为醇,包括以下步骤:第一步,在反应器中以升高的温度反应乙烯基不饱和化合物、一氧化碳、氢气和含膦的钴水合甲酰化催化剂,它们溶解在溶剂中;第二步,从包含催化剂和溶剂的溶液中分离出包含醇和重质组分的混合物;第三步,将溶液回收到反应器中。公开号:WO2004054946A1 -
作为产物:描述:参考文献:名称:Okita, Makoto; Wakamatsu, Takeshi; Mori, Miwako, Heterocycles, 1980, vol. 14, # 8, p. 1089 - 1092摘要:DOI:
文献信息
-
A Hydroformylation Route to Diazabicycloalkanes and Oxazabicycloalkanes Containing Medium and Large Rings作者:David J. Bergmann、Eva M. Campi、W. Roy Jackson、Antonio F. PattiDOI:10.1071/ch99097日期:——
Diazabicycloalkanes and oxazabicycloalkanes containing medium and large rings can be prepared by rhodium-catalysed reactions of N-alkenylpropane-1,3-diamines and 2-(alkenylamino)ethanols with H2/CO in excellent yields without the need for high dilution. Selective ring opening of these compounds can lead to large heterocycles.
-
[EN] NEW THIENOPYRIMIDINE DERIVATIVES, A PROCESS FOR THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING THEM<br/>[FR] NOUVEAUX DÉRIVÉS DE THIÉNOPYRIMIDINE, PROCÉDÉ POUR LEUR PRÉPARATION ET COMPOSITIONS PHARMACEUTIQUES LES CONTENANT申请人:SERVIER LAB公开号:WO2015097123A1公开(公告)日:2015-07-02Compounds of formula (I): wherein R1, R2, R3, R4, R5, R6, R7, R12, X, A and n are as defined in the description.式(I)的化合物:其中R1、R2、R3、R4、R5、R6、R7、R12、X、A和n的定义如描述中所述。
-
Quinazoline derivatives and pharmaceutical compositions containing them申请人:Zeneca Limited公开号:US06414148B1公开(公告)日:2002-07-02The invention relates to quinazoline derivatives of formula (1) wherein m is an integer from 1 to 2; R1 represents hydrogen, hydroxy, halogeno, nitro, trifluoromethyl, cyano, C1-3alkyl, C1-3alkoxy, C1-3alkylthio, or —NR5R6 (wherein R5 and R6, which may be the same or different, each represents hydrogen or C1-3alkyl); R2 represents hydrogen, hydroxy, halogeno, methoxy, amino or nitro; R3 represents hydroxy, halogeno, C1-3alkyl, C1-3alkoxy, C1-3alkanoyloxy, trifluoromethyl, cyano, amino or nitro; X1 represents —O—, —CH2—, —S—, —SO—, —SO2—, —NR7CO—, —CONR8—, —SO2NR9—, —NR10SO2— or —NR11— (wherein R7, R8, R9, R10 and R11 each independently represents hydrogen, C1-3alkyl or C1-3alkoxyC2-3alkyl); R4 represents an optionally substituted 5 or 6 membered saturated carbocyclic or heterocyclic group or a group which is alkenyl, alkynyl or optionally substituted alkyl, which alkyl group may contain a heteroatom linking group, which alkenyl, alkynyl or alkyl group may carry a terminal optionally substituted group selected from alkyl and a 5 or 6 membered saturated carbocyclic or heterocyclic group, and salts thereof; processes for their preparation, pharmaceutical compositions containing a compound of formula (I) or a pharmaceutically acceptable salt thereof as active ingredient. The compounds of formula (I) and pharmaceutically acceptable salts thereof inhibit the effects of VEGF, a property of value in the treatment of a number of disease states including cancer and rheumatoid arthritis.该发明涉及式(1)的喹唑啉衍生物, 其中m是1到2之间的整数;R1代表氢、羟基、卤素、硝基、三氟甲基、氰基、C1-3烷基、C1-3烷氧基、C1-3烷硫基,或-NR5R6(其中R5和R6,可以相同也可以不同,各自代表氢或C1-3烷基);R2代表氢、羟基、卤素、甲氧基、氨基或硝基;R3代表羟基、卤素、C1-3烷基、C1-3烷氧基、C1-3烷酰氧基、三氟甲基、氰基、氨基或硝基;X1代表-O-、-CH2-、-S-、-SO-、-SO2-、-NR7CO-、-CONR8-、-SO2NR9-、-NR10SO2-或-NR11-(其中R7、R8、R9、R10和R11各自独立地代表氢、C1-3烷基或C1-3烷氧基C2-3烷基);R4代表可选择地取代的5或6成员饱和碳环或杂环基团,或者是烯基、炔基或可选择地取代的烷基,该烷基可能含有连接基团的杂原子,该烯基、炔基或烷基基团可能携带一个末端可选择地取代的基团,所选自烷基和5或6成员饱和碳环或杂环基团,以及其盐;它们的制备方法,含有式(I)的化合物或其药学上可接受的盐作为活性成分的药物组合物。式(I)的化合物和其药学上可接受的盐抑制VEGF的作用,这是治疗多种疾病状态的一种有价值的特性,包括癌症和类风湿性关节炎。
-
SUBSTITUTED PYRIDINYL-PYRIMIDINES AND THEIR USE AS MEDICAMENTS申请人:Dahmann Georg公开号:US20130023502A1公开(公告)日:2013-01-24The invention relates to new substituted pyridinyl-pyrimidines of formula 1 wherein ring A is a five-membered saturated or unsaturated carbocyclic ring which optionally comprises one, two or three heteroatoms each independently from each other selected from the group N, S and O, wherein R 1 , R 2 , R 4 , R 3 , R 5 and R 6 are defined as in claim 1 and wherein ring A is further optionally substituted by one or two further substituents and the pharmaceutically acceptable salts, diastereomers, enantiomers, racemates, hydrates and solvates of the aforementioned compounds.
-
[EN] THIOPHENE COMPOUNDS<br/>[FR] COMPOSES DE BENZIMIDAZOLE-1-YL-THIOPHENE UTILISES EN CANCEROTHERAPIE申请人:SMITHKLINE BEECHAM CORP公开号:WO2004014899A1公开(公告)日:2004-02-19The present invention provides compounds of formula (I): (I) pharmaceutical compositions containing the same, processes for preparing the same and their use as pharmaceutical agents.本发明提供了式(I)的化合物: (I)含有相同化合物的药物组合物,制备该化合物的方法及其作为药物的应用。
表征谱图
-
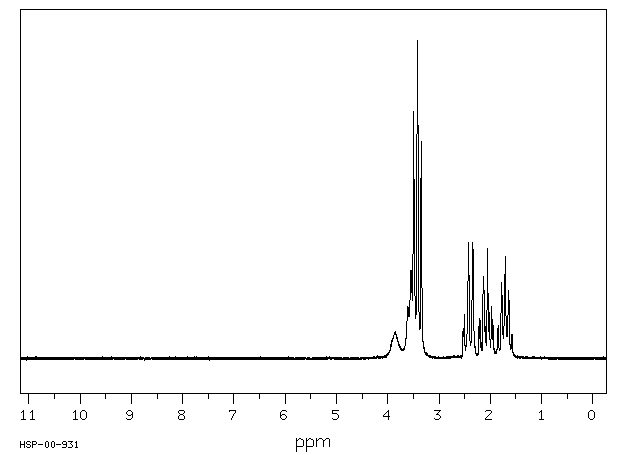
氢谱1HNMR
-
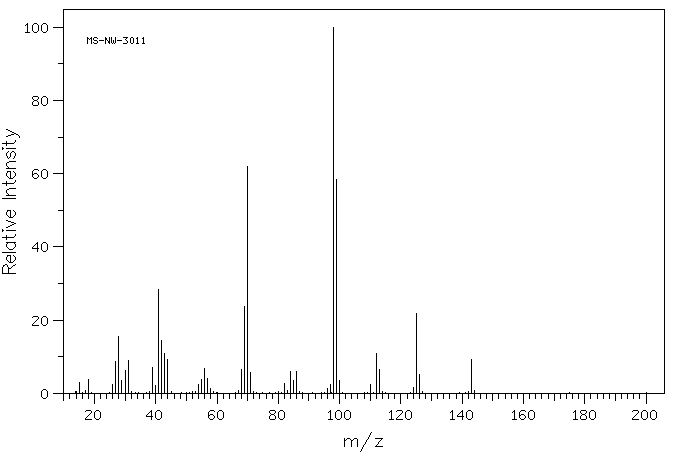
质谱MS
-
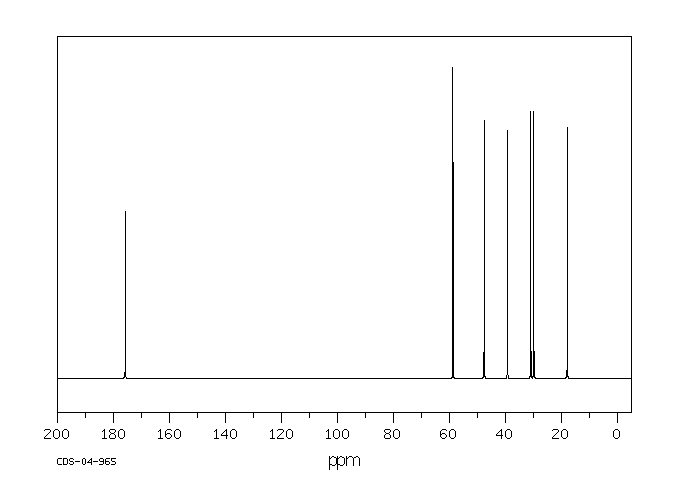
碳谱13CNMR
-
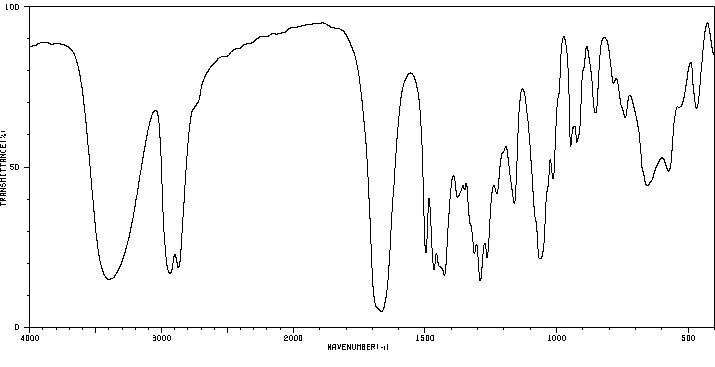
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5R,Z)-3-(羟基((1R,2S,6S,8aS)-1,3,6-三甲基-2-((E)-prop-1-en-1-yl)-1,2,4a,5,6,7,8,8a-八氢萘-1-基)亚甲基)-5-(羟甲基)-1-甲基吡咯烷-2,4-二酮
(2R,2''R)-(-)-2,2''-联吡咯烷
麦角甾-7,22-二烯-3-基亚油酸酯
马来酰亚胺霉素
马来酰亚胺基酰肼盐酸盐
马来酰亚胺基甲基-3-马来酰亚胺基丙酸酯
马来酰亚胺丙酰基-dPEG4-NHS
马来酰亚胺-酰胺-PEG6-琥珀酰亚胺酯
马来酰亚胺-酰胺-PEG6-丙酸
马来酰亚胺-酰胺-PEG24-丙酸
马来酰亚胺-酰胺-PEG12-丙酸
马来酰亚胺-四聚乙二醇-羧酸
马来酰亚胺-四聚乙二醇-丙酸叔丁酯
马来酰亚胺-四聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-六聚乙二醇-羧酸
马来酰亚胺-六聚乙二醇-丙酸叔丁酯
马来酰亚胺-八聚乙二醇-丙酸叔丁酯
马来酰亚胺-二聚乙二醇-丙酸叔丁酯
马来酰亚胺-三(乙烯乙二醇)-丙酸
马来酰亚胺-一聚乙二醇-羧酸
马来酰亚胺-一聚乙二醇-丙烯酸琥珀酰亚胺酯
马来酰亚胺-PEG3-羟基
马来酰亚胺-PEG2-胺三氟醋酸盐
马来酰亚胺-PEG2-琥珀酰亚胺酯
马来酰亚胺
频哪醇硼酸酯
顺式草酸双(-3,8-二氮杂双环[4.2.0]辛烷-8-羧酸叔丁酯)
顺式4-甲基吡咯烷酮-3-醇盐酸盐
顺式4-氟吡咯烷酮-3-醇盐酸盐
顺式3,4-二羟基吡咯烷盐酸盐
顺式3,4-二氨基吡咯烷-1-羧酸叔丁酯
顺式-二甲基 1-苄基吡咯烷-3,4-二羧酸
顺式-N-[2-(2,6-二甲基-1-哌啶基)乙基]-2-氧代-4-苯基-1-吡咯烷乙酰胺
顺式-N-Boc-吡咯烷-3,4-二羧酸
顺式-5-苄基-2-叔丁氧羰基六氢吡咯并[3,4-c]吡咯
顺式-5-甲基-1H-六氢吡咯并[3,4-b]吡咯二盐酸盐
顺式-5-氧代六氢环戊二烯并[c]吡咯-2(1H)-羧酸叔丁酯
顺式-5-乙氧羰基-1H-六氢吡咯并[3,4-B]吡咯盐酸盐
顺式-5-(碘甲基)-4-苯基-2-吡咯烷酮
顺式-5-(碘甲基)-4-甲基-2-吡咯烷酮
顺式-4-氧代-六氢-吡咯并[3,4-C]吡咯-2-甲酸叔丁酯
顺式-3-氟-4-羟基吡咯烷-1-羧酸叔丁酯
顺式-3-氟-4-甲基吡咯烷盐酸盐
顺式-2-甲基六氢吡咯并[3,4-c]吡咯
顺式-2,5-二甲基吡咯烷
顺式-1-苄基-3,4-吡咯烷二甲酸二乙酯
顺式-1-甲基六氢吡咯并[3,4-b]吡咯
顺式-(9CI)-3,4-二乙烯-1-(三氟乙酰基)-吡咯烷
顺-八氢环戊[c]吡咯-5-酮盐酸盐
非星匹宁