1-(3-羟丙基)-4-甲基哌嗪 | 5317-33-9
中文名称
1-(3-羟丙基)-4-甲基哌嗪
中文别名
1-(3-羟基丙基)-4-甲基哌嗪;1-(3羟丙基)-4-甲基哌嗪
英文名称
3-(4-methyl-1-piperazinyl)-1-propanol
英文别名
3-(4-methylpiperazin-1-yl)propan-1-ol;1-(3-hydroxypropyl)-4-methylpiperazine
CAS
5317-33-9
化学式
C8H18N2O
mdl
MFCD00009781
分子量
158.244
InChiKey
JKRSQNBRNIYETC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:30 °C
-
沸点:75-78°C/0.5mm
-
密度:0.988±0.06 g/cm3(Predicted)
-
溶解度:可溶于氯仿(少许)
计算性质
-
辛醇/水分配系数(LogP):-0.3
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:26.7
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2933599090
-
安全说明:S24/25
-
WGK Germany:3
-
危险标志:GHS07
-
危险性描述:H319
-
危险性防范说明:P305 + P351 + P338
-
储存条件:室温
SDS
| Name: | 3-(N-Methylpiperazine)-Propanol Material Safety Data Sheet |
| Synonym: | None known |
| CAS: | 5317-33-9 |
Synonym:None known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 5317-33-9 | 3-(N-Methylpiperazino)-Propanol | ca 100 | 226-177-6 |
Risk Phrases: 36/37/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes, respiratory system and skin.Corrosive.
Potential Health Effects
Eye:
Causes eye burns. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin burns. May cause skin rash (in milder cases), and cold and clammy skin with cyanosis or pale color.
Ingestion:
May cause severe and permanent damage to the digestive tract. Causes gastrointestinal tract burns. May cause perforation of the digestive tract. May cause systemic effects.
Inhalation:
Causes chemical burns to the respiratory tract. Aspiration may lead to pulmonary edema. May cause systemic effects. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Get medical aid immediately. Do NOT allow victim to rub eyes or keep eyes closed. Extensive irrigation with water is required (at least 30 minutes).
Skin:
Get medical aid immediately. Immediately flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Destroy contaminated shoes.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid immediately. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Get medical aid immediately. Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Do NOT use mouth-to-mouth resuscitation. If breathing has ceased apply artificial respiration using oxygen and a suitable mechanical device such as a bag and a mask.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use water spray to keep fire-exposed containers cool. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes.
Extinguishing Media:
Do NOT get water inside containers. For small fires, use dry chemical, carbon dioxide, or water spray. For large fires, use dry chemical, carbon dioxide, alcohol-resistant foam, or water spray.
Cool containers with flooding quantities of water until well after fire is out.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Avoid runoff into storm sewers and ditches which lead to waterways.
Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions. Absorb spill using an absorbent, non-combustible material such as earth, sand, or vermiculite. Do not use combustible materials such as sawdust.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use only in a well-ventilated area.
Do not get in eyes, on skin, or on clothing. Keep container tightly closed. Avoid ingestion and inhalation. Wash clothing before reuse.
Discard contaminated shoes.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances. Corrosives area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 5317-33-9: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: colorless, oily
Odor: none reported
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 120-121C @ 9mm Hg
Freezing/Melting Point: < 20 deg C
Autoignition Temperature: Not applicable.
Flash Point: > 109 deg C (> 228.20 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H18N2O
Molecular Weight: 158.1388
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents, strong acids, acid chlorides, acid anhydrides.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 5317-33-9 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-(N-Methylpiperazino)-Propanol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Products which are considered hazardous for supply are classified as Special Waste and the disposal of such chemicals is covered by regulations which may vary according to location. Contact a specialist disposal company or the local waste regulator for advice. Empty containers must be decontaminated before returning for recycling.
Section 14 - TRANSPORT INFORMATION
IATA
No information available.
IMO
No information available.
RID/ADR
No information available.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/37/38 Irritating to eyes, respiratory system
and skin.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 37/39 Wear suitable gloves and eye/face
protection.
WGK (Water Danger/Protection)
CAS# 5317-33-9: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 5317-33-9 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 5317-33-9 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-哌嗪基丙醇 3-(1-piperazinyl)-1-propanol 5317-32-8 C7H16N2O 144.217 4-甲基-1-哌嗪丙酸甲酯 methyl 3-(4-methylpiperazin-1-yl)propanoate 33544-40-0 C9H18N2O2 186.254 3-(4-甲基-哌嗪-1-基)-丙酸乙酯 ethyl 3-(4-methylpiperazin-1-yl)propanoate 7148-05-2 C10H20N2O2 200.281 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1,3-bis(4-methyl-piperazin-1-yl)propane —— C13H28N4 240.392 1-(2-羟乙基)-4-甲基哌嗪 2-(4-methylpiperazinyl)ethanol 5464-12-0 C7H16N2O 144.217
反应信息
-
作为反应物:描述:1-(3-羟丙基)-4-甲基哌嗪 在 palladium on activated charcoal 氢气 、 sodium 、 盐酸-N-乙基-Nˊ-(3-二甲氨基丙基)碳二亚胺 、 三乙胺 作用下, 以 四氢呋喃 、 甲醇 、 乙酸乙酯 、 N,N-二甲基甲酰胺 为溶剂, 20.0 ℃ 、413.7 kPa 条件下, 反应 7.0h, 生成 N-[4-[(3-methylphenyl)amino]-7-[3-N-(4-methylpiperazinyl)-propyloxy]-quinazolin-6-yl]-acrylamide参考文献:名称:酪氨酸激酶抑制剂。17.表皮生长因子受体的不可逆抑制剂:具有附加增溶功能的4-(苯基氨基)喹唑啉-和4-(苯基氨基)吡啶并[3,2-d]嘧啶-6-丙烯酰胺。摘要:通过使相应的6-胺与丙烯酸或丙烯酸酐反应,制备被可溶的7-烷基胺或7-烷氧基胺侧链取代的4-苯胺基喹唑啉-和4-苯胺基吡啶并[3,2-d]嘧啶-6-丙烯酰胺。在吡啶并[3,2-d]嘧啶系列中,中间体7-氨基-6-烷基胺是由7-溴-6-氟吡啶并[3,2-d]嘧啶在适当的条件下,通过钯与适当的锡烷偶联而制得的。 0)催化。这证明了引入阳离子增溶侧链的通用方法。评价化合物对分离的EGFR酶的磷酸化的抑制,以及对A431细胞中EGFR的EGF刺激的EGFR自身磷酸化的抑制以及MDA-MB 453细胞中调蛋白刺激的erbB2自身磷酸化的抑制。具有7-烷氧基胺增溶基团的喹唑啉类似物是分离的EGFR酶的有效不可逆抑制剂,IC(50p)值为2至4nM,并有效抑制细胞中的EGFR和erbB2自磷酸化。7-烷基氨基-和7-烷氧基氨基吡啶并[3,2-d]嘧啶也是不可逆的抑制剂,对分离的酶具有相同或更高的效力,但DOI:10.1021/jm990482t
-
作为产物:描述:参考文献:名称:Ratouis; Combes, Bulletin de la Societe Chimique de France, 1959, p. 576,578摘要:DOI:
-
作为试剂:描述:4-[(2,4-二氯-5-甲氧基苯基)氨基]-7-氟-6-甲氧基-3-喹啉甲腈 、 1-(3-羟丙基)-4-甲基哌嗪 在 1-(3-羟丙基)-4-甲基哌嗪 作用下, 以55的产率得到博舒替尼一水化合物参考文献:名称:[EN] 4-[(2,4-DICHLORO-5-METHOXYPHENYL)AMINO]-6-ALKOXY-3-QUINOLINECARBONITRILES FOR THE TREATMENT OF ISCHEMIC INJURY
[FR] 4-[(2,4-DICHLORO-5-METHOXYPHENYL)AMINO]-6-ALCOXY-3-QUINOLINECARBONITRILES POUR LE TRAITEMENT DES LESIONS ISCHEMIQUES摘要:公式(I)的化合物,其中X为N,CHn为1-3的整数;R'和R分别独立地表示1到3个碳原子的烷基,以及其药学上可接受的盐,但当n为1时,X不是N。这些化合物可用于抑制疾病、损伤或其他创伤引起的血管通透性。公开号:WO2004075898A1
文献信息
-
[EN] NEW THIENOPYRIMIDINE DERIVATIVES, A PROCESS FOR THEIR PREPARATION AND PHARMACEUTICAL COMPOSITIONS CONTAINING THEM<br/>[FR] NOUVEAUX DÉRIVÉS DE THIÉNOPYRIMIDINE, PROCÉDÉ POUR LEUR PRÉPARATION ET COMPOSITIONS PHARMACEUTIQUES LES CONTENANT申请人:SERVIER LAB公开号:WO2015097123A1公开(公告)日:2015-07-02Compounds of formula (I): wherein R1, R2, R3, R4, R5, R6, R7, R12, X, A and n are as defined in the description.式(I)的化合物:其中R1、R2、R3、R4、R5、R6、R7、R12、X、A和n的定义如描述中所述。
-
[EN] NOVEL PYRROLE DERIVATIVES<br/>[FR] NOUVEAUX DÉRIVÉS DE PYRROLE申请人:UNIV LEICESTER公开号:WO2014195705A1公开(公告)日:2014-12-11There are provided inter alia compounds of formula (I), wherein R1, R2, R3, R4a and R4b are as defined in the specification and their use in therapy, especially in the treatment of bacterial (e.g. pneumococcal) infections.其中提供了式(I)的化合物,其中R1、R2、R3、R4a和R4b如规范中定义,并且它们在治疗中的用途,特别是在细菌感染(例如肺炎球菌感染)的治疗中。
-
2-[1H-Benzimidazol-2(3H)-ylidene]-2-(pyrimidin-2-yl)acetamides and 2-[benzothiazol-2(3H)-ylidene]-2-(pyrimidin-2-yl)acetamides as kinase inhibitors申请人:Aurrecoechea Natalia公开号:US20100081653A1公开(公告)日:2010-04-012-[1H-benzimidazol-2(3H)-ylidene]-2-(pyrimidin-2-yl)acetamides and 2-[benzothiazol-2(3H)-ylidene]-2-(pyrimidin-2-yl)acetamides and their salts are kinase inhibitors, useful in the treatment of cancer.
-
Manganese-Catalyzed Anti-Markovnikov Hydroamination of Allyl Alcohols via Hydrogen-Borrowing Catalysis作者:Kuhali Das、Koushik Sarkar、Biplab MajiDOI:10.1021/acscatal.1c01199日期:2021.6.18In this article, a selective formal anti-Markovnikov hydroamination of allyl alcohols is presented. It enables the versatile synthesis of valuable γ-amino alcohol building blocks. A phosphine-free Earth’s abundant manganese(I) complex catalyzed the reaction under hydrogen-borrowing conditions. A vast range of aliphatic, aromatic amines, drug molecules, and natural product derivatives underwent successful
-
Derivatives of Amphotericin B申请人:Revolution Medicines, Inc.公开号:US20160304548A1公开(公告)日:2016-10-20Disclosed are derivatives of amphotericin B (AmB) characterized by improved therapeutic index compared to AmB. The AmB derivatives include C16 ureas, carbamates, and amides according to Formula (I); C3′-substituted C16 ureas, carbamates, and amides according to Formula (II); C16 acyls according to Formula (III); C2′epi-C16 ureas, carbamates, and amides according to Formula (IV); and C16 oxazolidinone derivatives according to Formula (V). Also disclosed are pharmaceutical compositions comprising the AmB derivatives, and therapeutic methods of using the AmB derivatives.
表征谱图
-
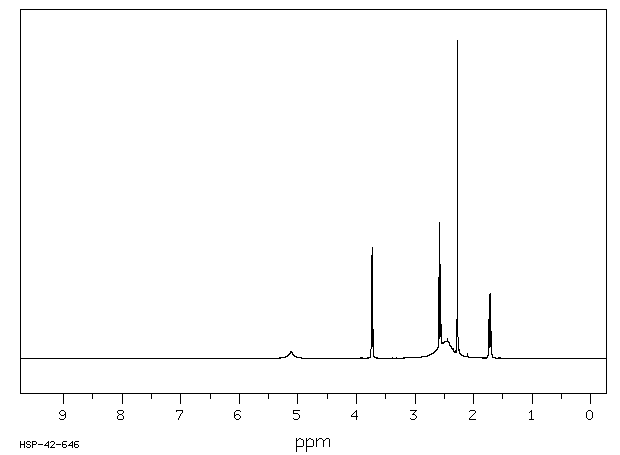
氢谱1HNMR
-
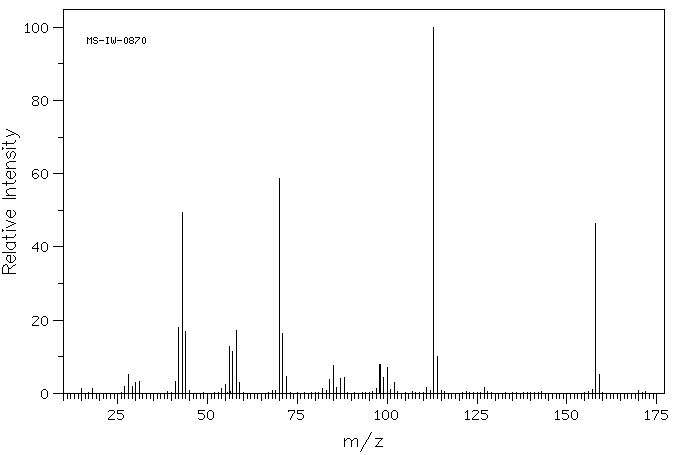
质谱MS
-
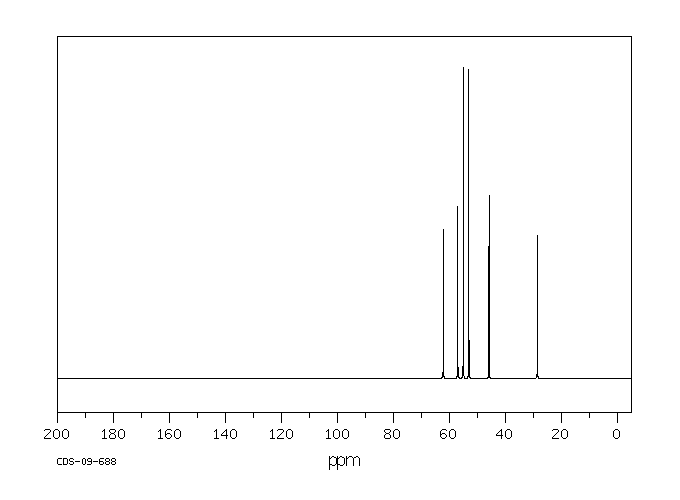
碳谱13CNMR
-
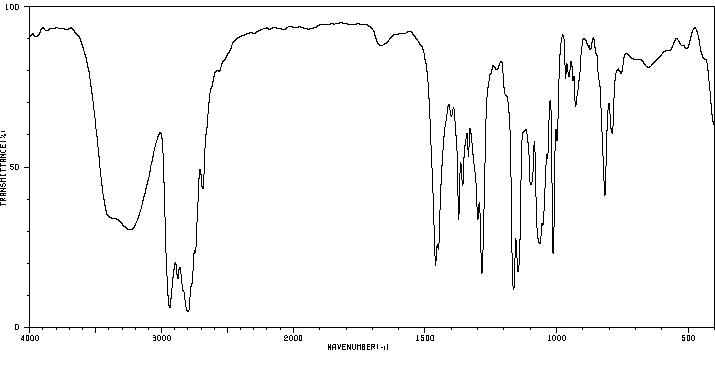
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2S)-4-[7-(8-氯-1-萘)-5,6,7,8-四氢-2-[[((2S)-1-甲基-2-吡咯烷基]甲氧基]吡啶基[3,4-d]嘧啶-4-基]-1-(2-氟-1-氧代-2-丙烯-1-基)-2-哌Chemicalbook嗪乙腈;2-((S)-4-(7-(8-氯萘-1-基)-2-((((S)-1-
齐拉西酮相关物质C
齐拉西酮杂质E
齐拉西酮开环物,氨基酸杂质
齐拉西酮亚砜
齐拉西酮 盐酸盐 一水合物
齐拉西酮
鲸蜡硬脂醇
鲁拉西酮杂质3
鲁拉西酮杂质23
鲁拉西酮杂质14
鲁拉西酮杂质11
鲁拉西酮
鲁拉西杂质E
鲁拉西杂质1
高分子量聚合三嗪类无卤阻燃剂
驱虫灵D
马福拉嗪
马来酸阿伐曲泊帕
顺式-1-乙酰基-2,6-二甲基-4-亚硝基-哌嗪
雷诺嗪双(N-氧化物)
陶扎色替
阿达色林
阿莫西林二氧代哌嗪
阿立哌唑羟基丁基杂质
阿立哌唑杂质26
阿立哌唑USP相关物质H
阿立哌唑N4-氧化物
阿立哌唑N,N-二氧化物
阿立哌唑EP杂质D
阿立哌唑-d8
阿立哌唑
阿泊替尼
阿替韦啶
阿拉诺丁
阿扎哌醇
阿得巴司
阿尔哌汀
阿伐曲泊帕杂质
间羟基苯基哌嗪
钾 1-甲基-4-三氟硼酸三甲基哌嗪
钠4-(4-乙酰基-1-哌嗪基)苯酚
酮齐拉西酮
酮酮唑油酸酯
酚酞单磷酸酯二-(2-氨基-2-甲基-1,3-丙二醇)盐
选择性氟试剂II
达鲁舍替
达哌唑
赫普索
赤霉素A7甲酯