松节油(萜烯、类萜物)月桂烯馏分羟基乙酸酯 | 1118-39-4
物质功能分类
中文名称
松节油(萜烯、类萜物)月桂烯馏分羟基乙酸酯
中文别名
2-甲基-6-亚甲基-7-辛烯-2-醇乙酸酯
英文名称
myrcenyl acetate
英文别名
Myrcenylacetat;(2-methyl-6-methylideneoct-7-en-2-yl) acetate
CAS
1118-39-4
化学式
C12H20O2
mdl
MFCD00087022
分子量
196.29
InChiKey
DCXXKSXLKWAZNO-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:273.19°C (rough estimate)
-
密度:0.9884 (rough estimate)
-
溶解度:Insoluble in water, soluble in alcohol and oils.
-
LogP:4.4 at 25℃
-
物理描述:Liquid
-
保留指数:1247;1246
-
稳定性/保质期:
- 该物质低毒、可燃,在加热分解时会释放出刺激性烟雾。
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:14
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.583
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
海关编码:2915390090
SDS
制备方法与用途
类别:有毒物品
毒性分级:低毒
急性毒性:
- 口服(大鼠)LD50:6300毫克/公斤
刺激数据:
- 皮肤(兔子):500毫克/24小时,中度
可燃性危险特性:
- 可燃;加热分解时释放刺激烟雾
储运特性:
- 库房通风、低温干燥
灭火剂:
- 干粉、泡沫、砂土和水
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:Ohloff,G. et al., Justus Liebigs Annalen der Chemie, 1964, vol. 675, p. 83 - 101摘要:DOI:
-
作为产物:描述:参考文献:名称:Schulze, Klaus; Haufe, Guenter; Koehler, Guenther, Zeitschrift fur Chemie, 1980, vol. 20, # 8, p. 293 - 294摘要:DOI:
文献信息
-
Iridium-catalyzed stereoselective [3+2] annulation of α-oxocarboxylic acids with 1,3-dienes作者:Ryota Yabe、Yusuke Ebe、Takahiro NishimuraDOI:10.1039/d1cc02003j日期:——The stereoselective annulation of α-oxocarboxylic acids with 1,3-dienes proceeded in the presence of a hydroxoiridium catalyst to give α-hydroxy-γ-lactones in good yields with high 3,5-trans relative stereochemistry. The use of a chiral diene ligand for a cationic iridium complex enabled asymmetric annulation with high enantioselectivity.
-
Ligand‐Regulated Regiodivergent Hydrosilylation of Isoprene under Iron Catalysis作者:Chang‐Sheng Kuai、Ding‐Wei Ji、Chao‐Yang Zhao、Heng Liu、Yan‐Cheng Hu、Qing‐An ChenDOI:10.1002/anie.202007930日期:2020.10.19feedstock isoprene with unactivated silanes has been developed using earth‐abundant iron catalysts. The manipulation of regioselectivity relies on fine modification of the coordination geometry of the iron center. While a bidentate pyridine imine ligand promoted the formation of allylic silanes through 4,1‐addition, selectivity for the 3,4‐adduct homoallylic silanes was observed with a tridentate nitrogen
-
Orthogonal Regulation of Nucleophilic and Electrophilic Sites in Pd‐Catalyzed Regiodivergent Couplings between Indazoles and Isoprene作者:Wen‐Shuang Jiang、Ding‐Wei Ji、Wei‐Song Zhang、Gong Zhang、Xiang‐Ting Min、Yan‐Cheng Hu、Xu‐Liang Jiang、Qing‐An ChenDOI:10.1002/anie.202100137日期:2021.4.6multiple active sites. Herein, an orthogonal regulation of nucleophilic and electrophilic sites in the regiodivergent hydroamination of isoprene with indazoles is demonstrated. Under Pd‐hydride catalysis, the 1,2‐ or 4,3‐insertion pathway with respect to the electrophilic sites on isoprene could be controlled by the choice of ligands. In terms of the nucleophilic sites on indazoles, the reaction occurs
-
Ruthenium-Catalyzed Cross Metathesis of β-Myrcene and its Derivatives with Methyl Acrylate作者:Arno Behr、Leif Johnen、Andreas Wintzer、Arzu Gümüş Çetin、Peter Neubert、Lutz DomkeDOI:10.1002/cctc.201500993日期:2016.2β‐myrcene with methyl acrylate produces 3‐methylenecyclopent‐1‐ene in the presence of the Ru catalyst Neolyst M2 by ring‐closing metathesis in the first step. In the second step, the generated methylidene unit reacts with methyl acrylate to give the corresponding unsaturated cyclic ester by cross metathesis. Under the optimized reaction conditions (80 °C, Neolyst M2, 16 equivalents of methyl acrylate), derivatives
-
Active-releasing cyclic siloxanes申请人:Perry J. Robert公开号:US20050136021A1公开(公告)日:2005-06-23Cyclic siloxanes that contain releasable active ingredients are described. The active ingredient can be an alcohol or enolizable carbonyl-containing compound such as a ketone, aldehyde, or ester. The product siloxanes are useful in a variety of personal and household care products where slow or controlled release of active ingredient is desired. A preferred embodiment utilizes substituents that when released as active ingredients are fragrant.
表征谱图
-
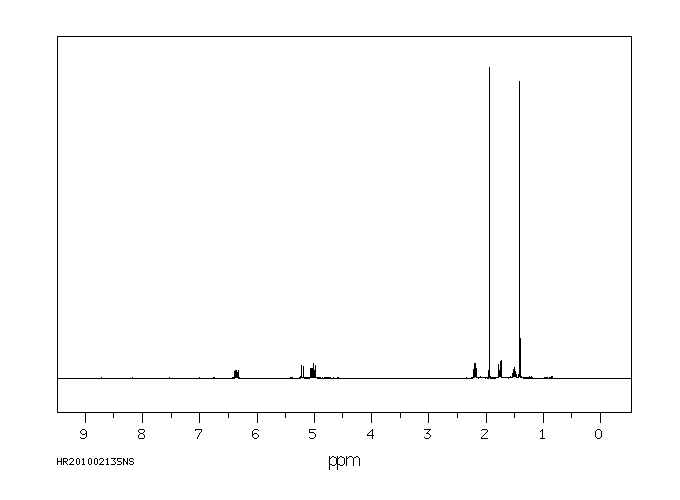
氢谱1HNMR
-
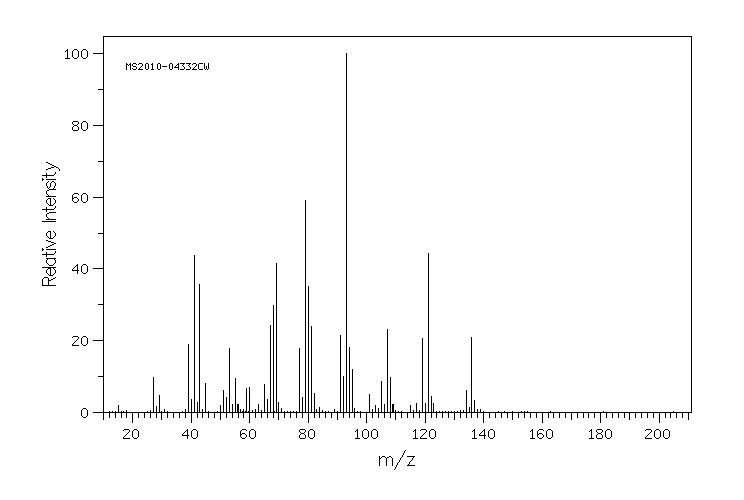
质谱MS
-
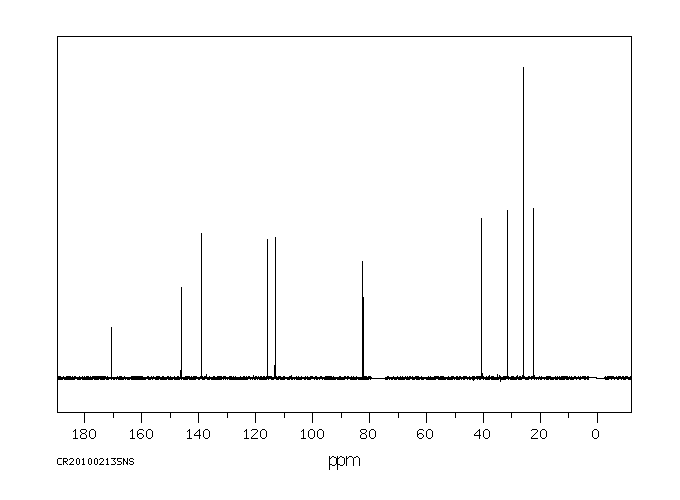
碳谱13CNMR
-
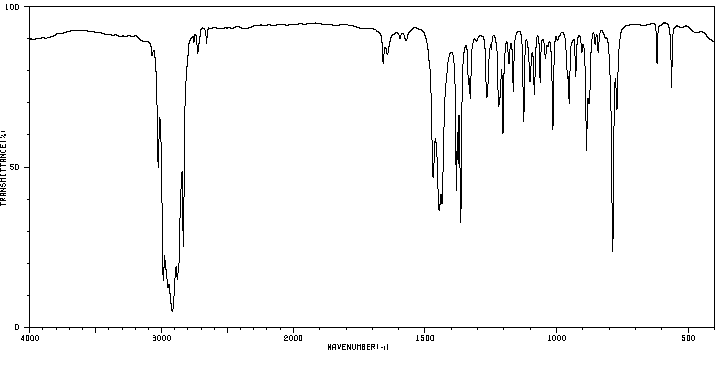
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸