1-乙酰吲哚 | 576-15-8
中文名称
1-乙酰吲哚
中文别名
N-乙酰基吲哚;1-乙酰基吲哚;N-乙醯吲哚
英文名称
N-acetylindole
英文别名
1-acetylindole;1-(1H-indol-1-yl)ethan-1-one;1-(1H-indol-1-yl)ethanone;1-indol-1-ylethanone
CAS
576-15-8
化学式
C10H9NO
mdl
MFCD00038005
分子量
159.188
InChiKey
UUCUQJHYUPXDHN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:189 °C
-
沸点:123-125 °C/8 mmHg (lit.)
-
密度:1.387 g/mL at 25 °C (lit.)
-
闪点:>230 °F
-
LogP:2.239 (est)
-
保留指数:1530;1487;1487
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):2.6
-
重原子数:12
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.1
-
拓扑面积:22
-
氢给体数:0
-
氢受体数:1
安全信息
-
WGK Germany:3
-
海关编码:29339990
-
安全说明:S24/25
-
储存条件:密封储存,应存放在阴凉干燥的库房中。
SDS
| Name: | 1-Acetylindole 98% Material Safety Data Sheet |
| Synonym: | None Known |
| CAS: | 576-15-8 |
Synonym:None Known
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 576-15-8 | 1-Acetylindole | 98% | 209-396-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: In case of contact, immediately flush eyes with plenty of water for at least 15 minutes. Get medical aid.
Skin:
In case of contact, flush skin with plenty of water. Remove contaminated clothing and shoes. Get medical aid if irritation develops and persists. Wash clothing before reuse.
Ingestion:
If swallowed, do not induce vomiting unless directed to do so by medical personnel. Never give anything by mouth to an unconscious person. Get medical aid.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Use foam, dry chemical, or carbon dioxide.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a cool, dry place. Store in a tightly closed container.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 576-15-8: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear to light yellow
Odor: characteristic odor
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 123 - 125 deg C @ 78mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 110 deg C (> 230.00 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.3870g/cm3
Molecular Formula: C10H9NO
Molecular Weight: 159.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 576-15-8 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-Acetylindole - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
IMO
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing Group:
RID/ADR
Shipping Name: Not regulated.
Hazard Class:
UN Number:
Packing group:
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 576-15-8: No information available.
Canada
CAS# 576-15-8 is listed on Canada's NDSL List.
CAS# 576-15-8 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 576-15-8 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1-乙酰基-5-溴吲哚 N-acetyl-5-bromoindole 61995-52-6 C10H8BrNO 238.084 N-乙酰基-3-羟基吲哚 1-(3-hydroxy-1H-indol-1-yl)ethanone 33025-60-4 C10H9NO2 175.187 —— N-benzoylindole 1496-76-0 C15H11NO 221.258 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-乙基吲哚 N-Ethylindole 10604-59-8 C10H11N 145.204 —— 1-isopropenyl-1H-indole 405150-33-6 C11H11N 157.215 1-(2-甲基-1H-吲哚-1-基)乙酮 1-acetyl-2-methylindole 37842-85-6 C11H11NO 173.214 1-(3-溴-1H-吲哚-1-基)乙酮 1-(3-bromo-1H-indol-1-yl)ethan-1-one 66417-73-0 C10H8BrNO 238.084 —— 1-(3-chloro-1H-indol-1-yl)ethan-1-one 94812-07-4 C10H8ClNO 193.633 —— 1-acetyl-3-fluoroindole 1245481-18-8 C10H8FNO 177.178 —— 1,6-diacetylindole 155703-10-9 C12H11NO2 201.225 (9ci)-1-乙酰基-6-(氯乙酰基)-1H-吲哚 1-Acetyl-6-chloroacetylindole 92013-04-2 C12H10ClNO2 235.67 N-乙酰基吲哚-3-甲醛 1-acetyl-3-indolylcarbaldehyde 22948-94-3 C11H9NO2 187.198 —— 1-acetyl-1H-indole-3-carbonitrile 861551-94-2 C11H8N2O 184.197 —— 1-acetyl-1H-3-indolyl thiocyanate 28733-72-4 C11H8N2OS 216.263 —— 1-acetyl-3-phenylindole 54470-44-9 C16H13NO 235.285 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:使用氯铬酸吡啶和可重复使用的聚苯胺盐催化剂组合氧化吲哚摘要:本文描述了一种新方法,用于在室温下或在二氯乙烷中回流,在聚苯胺盐催化剂的帮助下,使用氯铬酸吡啶鎓将吲哚简单、方便和有效地氧化为靛红。有趣的是,通过该程序氧化 3-烷基吲哚得到 3-羟基 3-烷基羟吲哚。另一方面,吲哚-3-烷醇产生靛红和3-甲酰基吲哚的混合物。这是第一个使用聚苯胺作为氧化反应催化剂的例子。DOI:10.1055/s-2008-1077974
-
作为产物:描述:2-碘乙酰苯胺 在 bis-triphenylphosphine-palladium(II) chloride 、 copper(l) iodide 、 C31H44N3P2Ru(1+)*F6P(1-) 、 potassium carbonate 、 三乙胺 作用下, 以 四氢呋喃 、 2-甲基四氢呋喃 、 甲醇 为溶剂, 反应 11.0h, 生成 1-乙酰吲哚参考文献:名称:Optimizing ligand structure for low-loading and fast catalysis for alkynyl-alcohol and -amine cyclization摘要:
对于一系列的[Ru(Cp/Cp*)(PR2NR′2)(MeCN)]PF6配合物进行了催化性能评估,其中初级配位球体的立体和电子特性发生了变化(R = Ph,
t -Bu, Bn; 以及Cpvs . Cp*)。DOI:10.1039/c9dt01870k
文献信息
-
Regioselective C–H Thioarylation of Electron-Rich Arenes by Iron(III) Triflimide Catalysis作者:Amy C. Dodds、Andrew SutherlandDOI:10.1021/acs.joc.1c00448日期:2021.4.16iron(III) triflimide allowed the efficient thiolation of a range of arenes, including anisoles, phenols, acetanilides, and N-heterocycles. The method was applicable for the late-stage thiolation of tyrosine and tryptophan derivatives and was used as the key step for the synthesis of pharmaceutically relevant biaryl sulfur-containing compounds such as the antibiotic dapsone and the antidepressant vortioxetine
-
N-2-嘧啶基-3-氟吲哚类化合物及其制备方法与应用申请人:浙江工业大学公开号:CN112574180A公开(公告)日:2021-03-30
-
The copper(<scp>ii</scp>)-catalyzed and oxidant-promoted regioselective C-2 difluoromethylation of indoles and pyrroles作者:Dong Zhang、Zheng Fang、Jinlin Cai、Chengkou Liu、Wei He、Jindian Duan、Ning Qin、Zhao Yang、Kai GuoDOI:10.1039/d0cc03345f日期:——highly selective C-2 difluoromethylation of indole derivatives was developed by using sodium difluoromethylsulfinate (HCF2SO2Na) as the source of difluoromethyl groups and a Cu(II) complex as the catalyst. Various substrates were well tolerated in this transformation and the desired products were obtained in moderate to good yields. Moreover, the late-stage C-2 difluoromethylation of bioactive molecules
-
Synthesis of difluoromethylated diarylmethanes <i>via</i> Fe(OTf)<sub>3</sub>-catalyzed Friedel–Crafts reaction of 2,2-difluoro-1-arylethyl phosphates作者:Yoshihiko Yamamoto、Tomoya Takase、Eisuke Kuroyanagi、Takeshi YasuiDOI:10.1039/d1cc00765c日期:——Fe(OTf)3-catalyzed Friedel–Crafts reaction of 2,2-difluoro-1-arylethyl phosphates with electron-rich (hetero)arenes afforded difluoromethylated diarylmethanes. Control experiments showed that Fe(OTf)3 behaves as the Lewis acid, and that the phosphate leaving group and o- or p-alkoxy substituents on the substrates are necessary for the Fe(OTf)3-catalyzed reaction to proceed under mild conditions.
-
Stereoselective Synthesis of Vinylcyclopropa[<i>b</i>]indolines via a Rh-Migration Strategy作者:Pan Guo、Wangbin Sun、Yu Liu、Yong-Xin Li、Teck-Peng Loh、Yaojia JiangDOI:10.1021/acs.orglett.0c02071日期:2020.8.7A mild rhodium catalytic system has been developed to synthesize vinylcyclopropa[b]indolines through cyclopropanation of indoles with vinyl carbenoids generated from ring opening of cyclopropenes in situ. By employing a Rh-migration strategy, the products can be obtained with good to excellent E:Z ratios (≤99:1) and complete diastereoselectivity (≤99:1). This method is easy, has a low catalyst loading
表征谱图
-
氢谱1HNMR
-
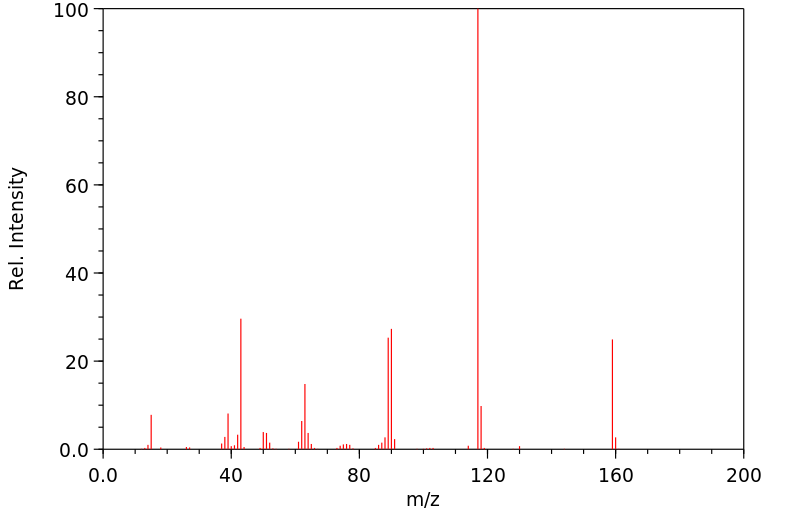
质谱MS
-
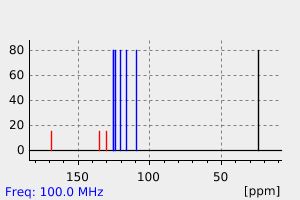
碳谱13CNMR
-
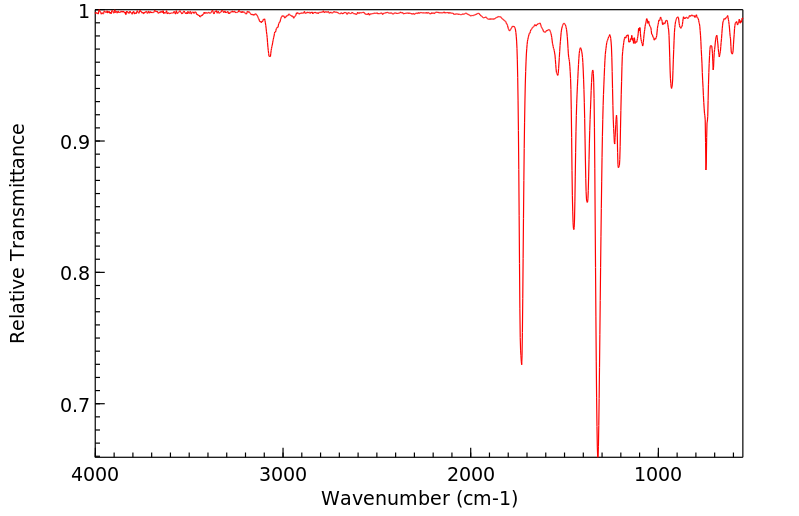
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3