间溴三氟甲苯 | 401-78-5
中文名称
间溴三氟甲苯
中文别名
间三氟甲基溴苯;3-溴三氟甲苯;间溴三氟甲基苯;1-溴-3-(三氟甲基)苯;3-溴-α,α,α-三氟甲苯;3-溴三氟甲基苯
英文名称
3-bromo-1-trifluoromethylbenzene
英文别名
3-Bromobenzotrifluoride;1-bromo-3-(trifluoromethyl)benzene;3-(trifluoromethyl)bromobenzene;m-bromotrifluorotoluene
CAS
401-78-5
化学式
C7H4BrF3
mdl
MFCD00000380
分子量
225.008
InChiKey
NNMBNYHMJRJUBC-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:1°C
-
沸点:151-152 °C(lit.)
-
密度:1.613 g/mL at 25 °C(lit.)
-
闪点:110 °F
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:11
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.142
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:3
安全信息
-
TSCA:Yes
-
危险等级:3
-
危险品标志:Xi
-
安全说明:S16,S23,S24/25,S26,S37/39
-
危险类别码:R10,R36/37/38
-
WGK Germany:3
-
海关编码:29036990
-
危险品运输编号:UN 1993 3/PG 3
-
危险类别:3
-
RTECS号:XS7970000
-
包装等级:III
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H225,H315,H319,H335
-
储存条件:本品应密封存放在阴凉处,并采用250kg内衬铁桶进行包装。
SDS
| Name: | 3-Bromobenzotrifluoride 99% Material Safety Data Sheet |
| Synonym: | m-Bromobenzotrifluoride; Benzene, 1-bromo-3-(trifluoromethyl) |
| CAS: | 401-78-5 |
Synonym:m-Bromobenzotrifluoride; Benzene, 1-bromo-3-(trifluoromethyl)
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 401-78-5 | 3-Bromobenzotrifluoride | 99 | 206-932-6 |
Risk Phrases: 10 36/38
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Flammable. Irritating to eyes and skin.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis and corneal damage.
Skin:
Causes skin irritation. May cause cyanosis of the extremities.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea. Ingestion of large amounts may cause CNS depression.
Inhalation:
May cause respiratory tract irritation. Aspiration may lead to pulmonary edema. Vapors may cause dizziness or suffocation. Can produce delayed pulmonary edema. May cause burning sensation in the chest.
Chronic:
Prolonged or repeated skin contact may cause dermatitis. Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. Vapors may form an explosive mixture with air.
Vapors can travel to a source of ignition and flash back. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Will burn if involved in a fire. Use water spray to keep fire-exposed containers cool. Containers may explode in the heat of a fire. Flammable liquid and vapor.
Extinguishing Media:
For small fires, use dry chemical, carbon dioxide, water spray or alcohol-resistant foam. For large fires, use water spray, fog, or alcohol-resistant foam. Use water spray to cool fire-exposed containers. Water may be ineffective. Do NOT use straight streams of water.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Remove all sources of ignition. Use a spark-proof tool. Provide ventilation. A vapor suppressing foam may be used to reduce vapors.
Section 7 - HANDLING and STORAGE
Handling:
Remove contaminated clothing and wash before reuse. Use only in a well-ventilated area. Ground and bond containers when transferring material. Use spark-proof tools and explosion proof equipment. Avoid contact with eyes, skin, and clothing. Empty containers retain product residue, (liquid and/or vapor), and can be dangerous. Keep container tightly closed. Keep away from heat, sparks and flame.
Avoid ingestion and inhalation. Do not pressurize, cut, weld, braze, solder, drill, grind, or expose empty containers to heat, sparks or open flames.
Storage:
Keep away from heat, sparks, and flame. Keep away from sources of ignition. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Flammables-area.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate general or local explosion-proof ventilation to keep airborne levels to acceptable levels.
Exposure Limits CAS# 401-78-5: United States OSHA: 2.5 mg/m3 TWA (as F) (listed under Fluorides Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear slightly yellow
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 151 - 152 deg C @ 760.00 mmHg
Freezing/Melting Point: Not available.
Autoignition Temperature: Not applicable.
Flash Point: 43 deg C ( 109.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.6130 g/cm3
Molecular Formula: C7H4BrF3
Molecular Weight: 225.01
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, ignition sources, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, hydrogen fluoride gas, hydrogen bromide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 401-78-5: XS7970000 LD50/LC50:
CAS# 401-78-5: Draize test, rabbit, eye: 500 mg/24H Mild; Draize test, rabbit, skin: 500 mg/24H Mild.
Carcinogenicity:
3-Bromobenzotrifluoride - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Shipping Name: FLAMMABLE LIQUID, N.O.S.*
Hazard Class: 3
UN Number: 1993
Packing Group: III
IMO
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3.3
UN Number: 1993
Packing Group: III
RID/ADR
Shipping Name: FLAMMABLE LIQUID, N.O.S.
Hazard Class: 3
UN Number: 1993
Packing group: III
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 10 Flammable.
R 36/38 Irritating to eyes and skin.
Safety Phrases:
S 9 Keep container in a well-ventilated place.
S 16 Keep away from sources of ignition - No
smoking.
S 33 Take precautionary measures against static
discharges.
WGK (Water Danger/Protection)
CAS# 401-78-5: No information available.
Canada
CAS# 401-78-5 is listed on Canada's NDSL List.
CAS# 401-78-5 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 401-78-5 is listed on the TSCA inventory.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
化学性质
无色透明液体。熔点为1℃,沸点为153.5℃,在44-48℃时(1.33kPa)可挥发,闪点为43℃,相对密度为1.623,折射率为1.4347。该物质不溶于水,但能溶于乙醇和乙醚。
用途主要用于生产农药和医药产品,如合成减肥药物芬氟拉明等。此外,它还可作为医药和农药中间体。
生产方法-
三氟甲基苯直接溴化法
收率为80%。所需原料消耗定额为:三氟甲基苯(≥98%)686kg/t、溴素1142kg/t、铁粉200kg/t。 -
由间氨基三氟甲苯重氮化、置换而得
在搅拌条件下,将间氨基三氟甲苯缓慢加入氢溴酸中,温度控制在50℃以下。冷却后,加入亚硝酸钠溶液(温度控制在10℃以下),直至完成重氮化反应。随后将所得的重氮液加入煮沸的溴化亚铜和氢溴酸溶液内,并开始水蒸气蒸馏。有机物随水蒸气蒸出,分层取有机层,依次用水、稀碱液洗涤至中性,油层用无水氯化钙脱水后减压蒸馏,收集73-74.5℃(6.0kPa)的馏分,得间溴三氟甲苯。
有毒物品。
刺激数据- 皮肤:兔实验中,500毫克/24小时接触反应为轻度刺激。
- 眼睛:兔实验中,500毫克/24小时接触反应为中度刺激。
库房需保持通风、低温干燥环境。
灭火剂上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 对溴三氟甲苯 p-trifluoromethylphenyl bromide 402-43-7 C7H4BrF3 225.008 邻溴三氟甲苯 o-trifluoromethylphenyl bromide 392-83-6 C7H4BrF3 225.008 5-溴-2-碘三氟甲苯 4-bromo-1-iodo-2-(trifluoromethyl)benzene 364-12-5 C7H3BrF3I 350.905 三氟甲苯 α,α,α-trifluorotoluene 98-08-8 C7H5F3 146.112 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2-二溴-4-三氟甲苯 1,2-dibromo-4-(trifluoromethyl)benzene 7657-08-1 C7H3Br2F3 303.904 对溴三氟甲苯 p-trifluoromethylphenyl bromide 402-43-7 C7H4BrF3 225.008 3-溴-4-碘三氟甲苯 2-bromo-1-iodo-4-(trifluoromethyl)benzene 481075-58-5 C7H3BrF3I 350.905 3-溴-5-碘三氟甲苯 1-bromo-3-iodo-5-(trifluoromethyl)benzene 481075-59-6 C7H3BrF3I 350.905 5-溴-2-碘三氟甲苯 4-bromo-1-iodo-2-(trifluoromethyl)benzene 364-12-5 C7H3BrF3I 350.905 2-氨基-5-溴三氟甲苯 4-bromo-2-trifluoromethyl-aniline 445-02-3 C7H5BrF3N 240.023 三氟甲苯 α,α,α-trifluorotoluene 98-08-8 C7H5F3 146.112
反应信息
-
作为反应物:参考文献:名称:LiCl存在下三氟甲基苯基卤化镁的制备及2'-三氟甲基芳族酮的合成摘要:研究了LiCl促进镁屑插入卤代三氟甲基苯中用于格氏试剂合成的效果。在少于1.0当量的LiCl存在下,容易产生氯(三氟甲基)苯格氏试剂。高产率地获得2-三氟甲基苯基氯化镁格氏试剂,并且通过使所获得的格氏试剂与羧酸酐反应以83%的产率合成2'-三氟甲基取代的芳族酮。DOI:10.1021/acs.oprd.6b00200
-
作为产物:描述:参考文献:名称:使用 N-卤素化合物的卤化。二、1,3-二溴-5,5-二甲基乙内酰脲酸催化溴化芳香族化合物摘要:通过添加强酸促进在二氯甲烷中使用 1,3-二溴-5,5-二甲基乙内酰脲环溴化芳族化合物。pKa值低于-2的有机酸和无机酸均表现出促进作用。这种酸催化的溴化反应既实用又有效,即使对于具有吸电子取代基的芳烃也是如此。DOI:10.1246/bcsj.67.1918
-
作为试剂:参考文献:名称:[EN] PROCESS FOR THE PREPARATION OF FLIBANSERIN INVOLVING NOVEL INTERMEDIATES
[FR] PROCÉDÉ DE PRÉPARATION DE LA FLIBANSÉRINE IMPLIQUANT DE NOUVEAUX INTERMÉDIAIRES摘要:本发明涉及一种制备flibanserin的方法,其中涉及新型中间体。1,3-二氢-1-(2-溴乙基)-3-异丙烯基-2H-苯并咪唑-2-酮与二乙醇胺反应,并将所得的二羟基化合物转化为1,3-二氢-1-[2-[N-[双-(2-氯乙基)氨基]乙基]-3-异丙烯基-2H-苯并咪唑-2-酮,随后进行去保护作用,得到1,3-二氢-1-[2-[N-双-(2-氯乙基)氨基]乙基]-1,2-H-苯并咪唑-2-酮。这种化合物与m-三氟甲基苯胺缩合,得到flibanserin盐酸盐。公开号:WO2010128516A2
文献信息
-
Synthesis of 4-Substituted Chlorophthalazines, Dihydrobenzoazepinediones, 2-Pyrazolylbenzoic Acid, and 2-Pyrazolylbenzohydrazide via 3-Substituted 3-Hydroxyisoindolin-1-ones作者:Hanh Nho Nguyen、Victor J. Cee、Holly L. Deak、Bingfan Du、Kathleen Panter Faber、Hakan Gunaydin、Brian L. Hodous、Steven L. Hollis、Paul H. Krolikowski、Philip R. Olivieri、Vinod F. Patel、Karina Romero、Laurie B. Schenkel、Stephanie D. Geuns-MeyerDOI:10.1021/jo3000628日期:2012.4.20hydrazine, followed by chlorination with POCl3. We have also discovered two novel transformations of 3-vinyl- and 3-alkynyl-3-hydroxyisoindolinones. Addition of vinyl organometallic reagents to N,N-dimethylaminophthalimide (8a) provided dihydrobenzoazepinediones 15a–15c via the proposed ring expansion of 3-vinyl-3-hydroxyisoindolinone intermediates. 3-Alkynyl-3-hydroxyisoindolinones react with hydrazine and在本文中,我们描述了以良好的总收率进行的4-取代的氯邻苯二甲腈的一般三步合成。在关键步骤中,N,N-二甲基氨基邻苯二甲酰亚胺(8a)指导烷基,芳基和杂芳基有机金属试剂的选择性单加成反应,得到3-取代的3-羟基异吲哚满酮9b,9i - 9am。通过与肼反应,然后用POCl 3氯化,许多羟基异吲哚啉酮可转化为氯酞嗪1b - 1v。我们还发现了3-乙烯基和3-炔基-3-羟基异吲哚满酮的两个新颖的转化。将乙烯基有机金属试剂添加到N,N-二甲基氨基邻苯二甲酰亚胺(8a)通过提议的3-乙烯基-3-羟基异吲哚满酮中间体的扩环作用提供了二氢苯并氮杂氮杂二酮15a - 15c。3-炔基-3-羟基异吲哚啉酮与肼和取代的肼反应,生成2-吡唑基苯甲酸16a - 16d和2-吡唑基苯并酰肼17a - 17g,而不是预期的炔基酞菁。
-
Regioselective Arylation Reactions of Biphenyl-2-ols, Naphthols, and Benzylic Compounds with Aryl Halides under Palladium Catalysis作者:Tetsuya Satoh、Jun-ichi Inoh、Yoshiki Kawamura、Yuichiro Kawamura、Masahiro Miura、Masakatsu NomuraDOI:10.1246/bcsj.71.2239日期:1998.9Biphenyl-2-ols undergo regioselective mono- and diarylation upon a treatment with aryl iodides in the presence of a palladium catalyst in DMF using Cs2CO3 as a base to produce 1,1′ : 2′,1″-terphenyl-2-ol and 2′,6′-diphenylbiphenyl-2-ol and their derivatives. The reaction of 1-naphthol selectively occurs at its 8-position to give 8-aryl-1-naphthols. In the reaction of 2-naphthol with aryl bromides, diarylated
-
Palladium nanoparticles as reusable catalyst for the synthesis of <i>N</i>-aryl sulfonamides under mild reaction conditions作者:Mehdi Khalaj、Majid Ghazanfarpour-Darjani、Mohamad Reza Talei Bavil Olyai、Sakineh Faraji ShamamiDOI:10.1080/17415993.2015.1122010日期:2016.3.3An efficient palladium nanoparticles-catalyzed N-arylation of sulfonamides and sulfonyl azides is described. This procedure serves as an active protocol for intermolecular C–N bond formation using Pd(OAc)2 in PEG-400 under air. Aryl bromides and triflates react at 35°C, while aryl chlorides require heating to 50°C and give the desired products only in low yields. This reaction proceeds smoothly in
-
Nanocopper-mediated direct arylsulfonamidation of aryl halides with arylsulfonyl azides作者:I. Yavari、Y. Solgi、M. Ghazanfarpour-Darjani、S. AhmadianDOI:10.1007/s13738-012-0115-2日期:2012.12An efficient protocol for direct arylsulfonamidation of aryl iodides and aryl bromides using arylsulfonylazides catalyzed by copper nanoparticles is described.
-
Synthesis of Aromatic Carboxylic Acids by Carbonylation of Aryl Halides in the Presence of Epoxide-Modified Cobalt Carbonyls as Catalysts作者:V. P. Boyarskii、T. E. Zhesko、S. A. LaninaDOI:10.1007/s11167-005-0619-y日期:2005.11A new procedure was developed for synthesis of aromatic and heteroaromatic acids and their derivatives (esters, salts) by carbonylation of the corresponding aryl halides. The acids are selectively formed in a high yield under very mild conditions. Highly active catalytic systems, base-containing alcoholic solutions of cobalt carbonyl modified with epoxides, were used to activate aryl halides.
表征谱图
-
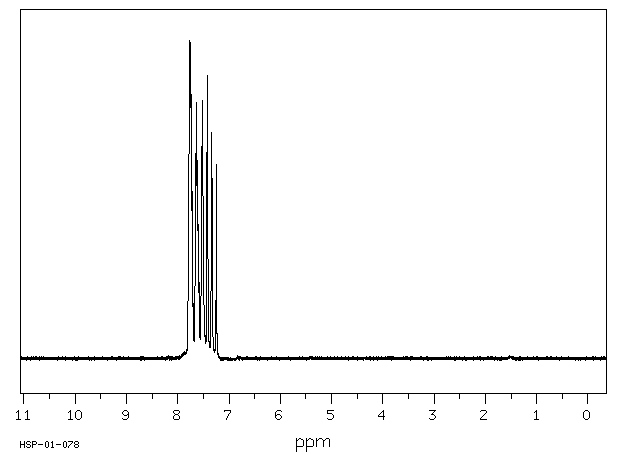
氢谱1HNMR
-
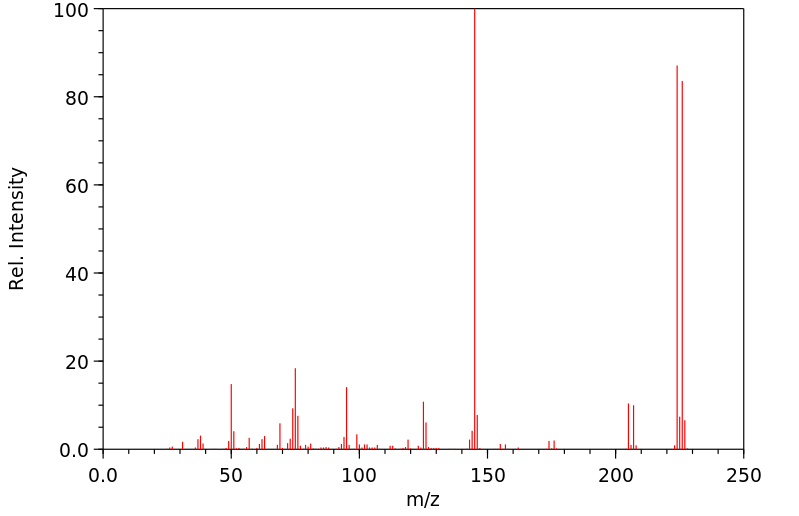
质谱MS
-
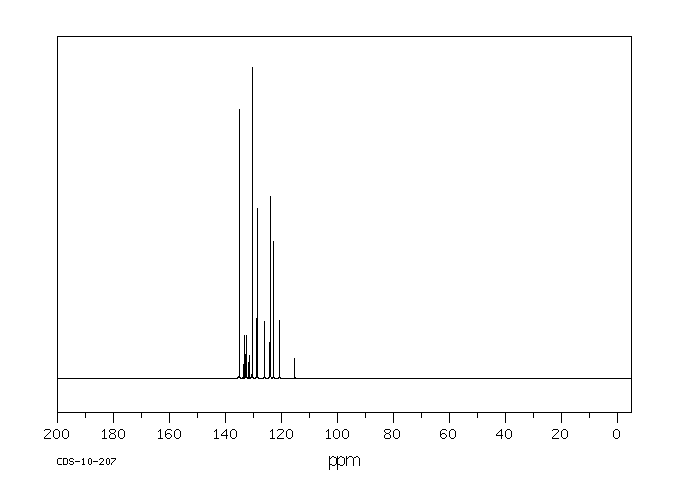
碳谱13CNMR
-
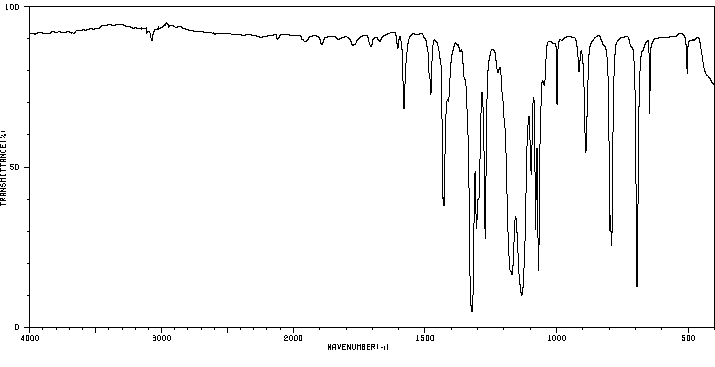
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫