2,2-二(2-四氢呋喃基)丙烷 | 89686-69-1
中文名称
2,2-二(2-四氢呋喃基)丙烷
中文别名
2,2-二(2-四氢化呋喃)丙烷;DTHFP
英文名称
2,2-bis(2-tetrahydrofuranyl)propane
英文别名
ditetrahydrofurylpropane;2,2-bis(2-tetrahydrofuryl)propane;DTHFP;2,2-bis(tetrahydrofuran-2-yl)propane;2,2-di(2-tetrahydrofuryl)propane;2-[2-(oxolan-2-yl)propan-2-yl]oxolane
CAS
89686-69-1
化学式
C11H20O2
mdl
MFCD00142763
分子量
184.279
InChiKey
FZLHAQMQWDDWFI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:146°C
-
密度:1 g/cm3
-
闪点:95°C(lit.)
-
LogP:2.7 at 22℃ and pH7
-
表面张力:69mN/m at 1.046g/L and 19.9℃
计算性质
-
辛醇/水分配系数(LogP):2.2
-
重原子数:13
-
可旋转键数:2
-
环数:2.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:18.5
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S16
-
危险类别码:R10
-
WGK Germany:3
-
危险品运输编号:UN 1993
SDS
2,2-Di(2-tetrahydrofuryl)propane
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2-Di(2-tetrahydrofuryl)propane
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 4
Acute toxicity (Dermal)
Acute toxicity (Inhalation) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed, in contact with skin or if inhaled
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF ON SKIN: Gently wash with plenty of soap and water.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2-Di(2-tetrahydrofuryl)propane
Percent: >96.0%(GC)
89686-69-1
CAS Number:
Chemical Formula: C11H20O2
2,2-Di(2-tetrahydrofuryl)propane
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
2,2-Di(2-tetrahydrofuryl)propane
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Form: Clear
Colour: Colorless - Almost colorless
Odour: Slight
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 146°C
Flash point: 95°C
Flammability or explosive
limits:
Lower: 0.7%
Upper: 6.3%
Vapour density: 6.3
Relative density: 1.00
Solubility(ies):
[Water] Very slightly soluble (0.13g/100mL, 20°C)
[Other solvents] No data available
Autoignition temperature: 190°C
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
2,2-Di(2-tetrahydrofuryl)propane
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 2,2-Di(2-tetrahydrofuryl)propane
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Acute toxicity (Oral) Category 4
Category 4
Acute toxicity (Dermal)
Acute toxicity (Inhalation) Category 4
Not classified
ENVIRONMENTAL HAZARDS
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Harmful if swallowed, in contact with skin or if inhaled
Precautionary statements:
Avoid breathing dust/fume/gas/mist/vapours/spray.
[Prevention]
Use only outdoors or in a well-ventilated area.
Do not eat, drink or smoke when using this product.
Wash hands thoroughly after handling.
Wear protective gloves and eye/face protection.
[Response] IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
breathing. Call a POISON CENTER or doctor/physician if you feel unwell.
IF SWALLOWED: Call a POISON CENTER or doctor/physician if you feel unwell.
Rinse mouth.
IF ON SKIN: Gently wash with plenty of soap and water.
Wash contaminated clothing before reuse.
Call a POISON CENTER or doctor/physician if you feel unwell.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 2,2-Di(2-tetrahydrofuryl)propane
Percent: >96.0%(GC)
89686-69-1
CAS Number:
Chemical Formula: C11H20O2
2,2-Di(2-tetrahydrofuryl)propane
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Call a POISON CENTER or doctor/physician if you feel unwell.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Call a POISON CENTER or doctor/physician if you feel unwell. Rinse mouth.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use personal protective equipment. Keep people away from and upwind of spill/leak.
Personal precautions,
protective equipment and Ensure adequate ventilation. Entry to non-involved personnel should be controlled
emergency procedures: around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Absorb spilled material in a suitable absorbent (e.g. rag, dry sand, earth, saw-dust).
containment and cleaning In case of large amount of spillage, contain a spill by bunding. Adhered or collected
up: material should be promptly disposed of, in accordance with appropriate laws and
regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent generation of vapour or mist. Wash hands and face thoroughly after
handling.
Use a ventilation, local exhaust if vapour or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Install a closed system or local exhaust as possible so that workers should not be
Engineering controls:
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Vapor respirator. Follow local and national regulations.
Protective gloves.
Hand protection:
Eye protection: Safety glasses. A face-shield, if the situation requires.
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Liquid
2,2-Di(2-tetrahydrofuryl)propane
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Form: Clear
Colour: Colorless - Almost colorless
Odour: Slight
pH: No data available
Melting point/freezing point:No data available
Boiling point/range: 146°C
Flash point: 95°C
Flammability or explosive
limits:
Lower: 0.7%
Upper: 6.3%
Vapour density: 6.3
Relative density: 1.00
Solubility(ies):
[Water] Very slightly soluble (0.13g/100mL, 20°C)
[Other solvents] No data available
Autoignition temperature: 190°C
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
No data available
Fish:
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
No data available
Soil adsorption (Koc):
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to burn in a chemical
incinerator equipped with an afterburner and scrubber system. Observe all federal, state and local regulations when
disposing of the substance.
2,2-Di(2-tetrahydrofuryl)propane
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
反应信息
-
作为反应物:描述:2,2-二(2-四氢呋喃基)丙烷 在 230-400 mesh size silica gel 作用下, 以 乙醚 、 正戊烷 为溶剂, 以20%的产率得到meso-2,2-di(2-tetrahydrofuryl)propane参考文献:名称:[EN] IMPROVED VINYL MODIFIER COMPOSITION AND PROCESSES FOR UTILIZING SUCH COMPOSITION
[FR] COMPOSITION AMÉLIORÉE DE MODIFICATION DE LA TENEUR EN VINYLE ET PROCÉDÉS D'UTILISATION D'UNE TELLE COMPOSITION摘要:提供了一种含有氧杂环戊烷化合物的组合物,其包含指定结构的一个或多个氧杂环戊烷化合物的中间异构体的指定量。还提供了使用这种组合物作为聚合过程中乙烯含量调节剂的方法。公式(I)公开号:WO2011087841A1 -
作为产物:描述:参考文献:名称:一种2,2-二(2-呋喃基)丙烷加氢制2,2-二(2- 四氢呋喃基)丙烷的方法摘要:本发明提供一种2,2‑二(2‑呋喃基)丙烷加氢制2,2‑二(2‑四氢呋喃基)丙烷的方法,其特征在于,所述方法包括以2,2‑二(2‑呋喃基)丙烷为原料,在使用复合催化剂催化的条件下与氢气发生加氢反应得到所述2,2‑二(2‑四氢呋喃基)丙烷,所述复合催化剂包括分布在催化剂载体上的活性组分、第一助剂和第二助剂,所述活性组分为金属Ru,所述第一助剂为金属Li、Na和K中的任意一种或多种,所述第二助剂为金属Fe、Co和Cu中的任意一种或多种。使用本发明中所述复合催化剂催化2,2‑二(2‑呋喃基)丙烷加氢,原料转化率和产物选择性高,同时产物的Meso异构体含量高,催化剂寿命长,该催化剂可以连续套用20次以上,仍保持很好加氢的活性。公开号:CN108997266B
-
作为试剂:描述:参考文献:名称:신규 리튬 화합물 및 이의 제조방법摘要:本发明提供了以下化学式1的新锂化合物及其制备方法:[化学式1] (在上述化学式1中,R到R,R和n的含义如文件中所定义)公开号:KR20170088515A
文献信息
-
Processes for preparing functionahzed polymers, related functionalizing compound and preparation thereof申请人:Bridgestone Corporation公开号:US10730961B2公开(公告)日:2020-08-04Disclosed herein are processes for preparing functionalized polymers, functionalizing compounds useful in the processes and processes for preparing the functionalizing compound. The processes for preparing a functionalized polymer include reaction of a functionalizing compound (prepared from the reaction of an alkoxysilane compound of formula (I) with a polyol of formula (II)) with a reactive conjugated diene monomer-containing polymer, thereby producing a polymer end functionalized with the functionalizing compound. In certain embodiments, the functionalizing compound has a formula according to formula (III), (IV), or (V).公开了用于制备官能化聚合物的过程、在过程中有用的官能化化合物以及制备官能化化合物的过程。制备官能化聚合物的过程包括使官能化化合物(由公式(I)的烷氧基硅烷化合物与公式(II)的多羟基醇反应制得)与包含反应性共轭二烯单体聚合物反应,从而产生以官能化化合物端基官能化的聚合物。在某些实施方式中,官能化化合物的公式符合公式(III)、(IV)或(V)。
-
METHOD FOR PRODUCING MODIFIED CONJUGATED DIENE-BASED POLYMER, MODIFIED CONJUGATED DIENE-BASED POLYMER, POLYMER COMPOSITION, CROSSLINKED BODY, TIRE AND COMPOUND申请人:JSR CORPORATION公开号:US20190194430A1公开(公告)日:2019-06-27A modified conjugated diene-based polymer having high Mooney viscosity and good shape stability and exhibiting excellent processability and low heat build-up is obtained in as few steps as possible. The modified conjugated diene-based polymer is produced by a method of reacting a conjugated diene-based polymer having an active chain end, obtained by polymerizing a monomer containing a conjugated diene compound in the presence of an initiator that contains at least either of an alkali metal compound and an alkaline earth metal compound, with a compound [M] having at least two groups selected from the group consisting of a group “—C(R 1 )N-A 1 ” and a group “—N═C(R 1 )-A 1 ”, where R 1 is a hydrogen atom or a hydrocarbyl group and A 1 is a monovalent group having an alkoxysilyl group.
-
MODIFIED STYRENE-BUTADIENE COPOLYMER, PREPARATION METHOD THEREOF, AND RUBBER COMPOSITION INCLUDING THE SAME申请人:LG Chem, Ltd.公开号:US20170204205A1公开(公告)日:2017-07-20The present invention relates to a modified styrene-butadiene copolymer with a high modification ratio, including a functional group derived from a modifier represented by Formula 1, a preparation method thereof, a rubber composition including the same, and a tire manufactured from the rubber composition.
-
[EN] METALLATED BENZYLSILANES AND THEIR USE AS POLYMERIZATION INITIATORS<br/>[FR] BENZYLSILANES MÉTALLÉS ET LEUR UTILISATION EN TANT QU'INITIATEURS DE POLYMÉRISATION申请人:TRINSEO EUROPE GMBH公开号:WO2018150043A1公开(公告)日:2018-08-23The invention relates to novel compounds useful as polymerization initiators or precursors for polymerization initiators. The invention further relates to a method of making the polymerization initiators and resulting polymers. The invention also relates to polymer compositions comprising the polymer of the invention and further components such as extender oils, fillers, vulcanizing agents etc., and to corresponding vulcanized polymer compositions and articles comprising vulcanized parts made from the vulcanized polymer composition.
-
Functionalized monomers for synthesis of rubbery polymers申请人:——公开号:US20040063884A1公开(公告)日:2004-04-01The present invention relates to a rubbery polymer which is comprised of repeat units that are derived from (1) at least one conjugated diolefin monomer, and (2) at least one functionalized monomer of the structural formula: 1 wherein the R′ groups in repeat units and in different repeat units can be the same or different and represent hydrogen atoms or alkyl groups containing from 1 to about 4 carbon atoms, wherein x represents an integer from 1 to about 10, and wherein the R groups in repeat units and in different repeat units can be the same or different and represent alkyl groups containing from 1 to about 10 carbon atoms or alkoxy groups containing from 1 to about 10 carbon atoms.本发明涉及一种橡胶聚合物,其由以下重复单元组成:(1)至少一种共轭二烯单体,和(2)至少一种结构式为的官能化单体,其中重复单元中的R′基和不同重复单元中的R′基可以相同或不同,代表氢原子或含有1至约4个碳原子的烷基基团,其中x代表从1到约10的整数,重复单元中的R基和不同重复单元中的R基可以相同或不同,代表含有1至约10个碳原子的烷基基团或含有1至约10个碳原子的烷氧基团。
表征谱图
-
氢谱1HNMR
-
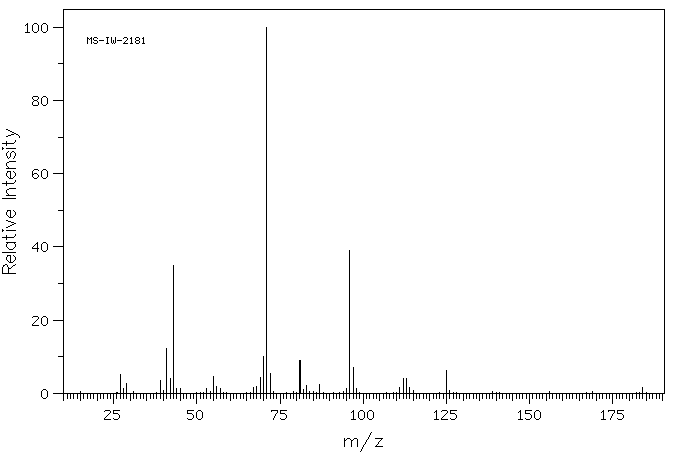
质谱MS
-
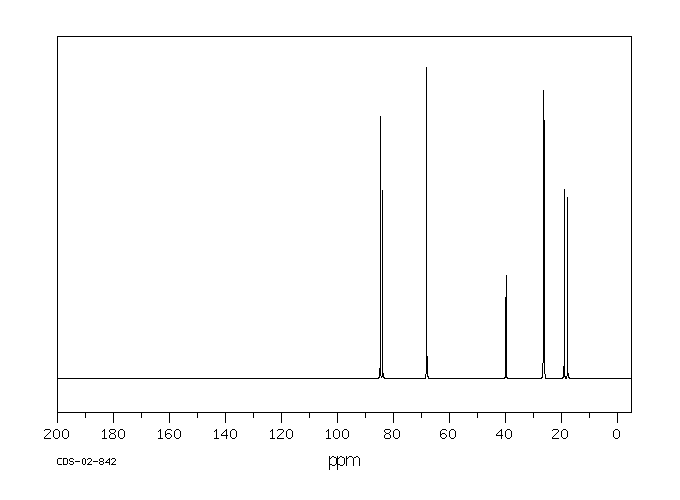
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-环氧丙烷-3,4-二胺二盐酸盐
顺式-2-(碘甲基)-3-羟基四氢呋喃
顺式-2-(碘甲基)-3-羟基四氢呋喃
青榄呋喃
茶香螺烷
苯胺,4-(2-哌嗪基)-
碳氯灵
硼烷四氢呋喃络合物
硫丹乙酯
甲基甲丙烯酰酸酯-叔丁基甲丙烯酰酸酯-月桂基甲丙烯酰酸酯共聚物
甲基丙烯酸四氢糠基酯
甲基[(噁戊环-2-基)甲基]胺盐酸
甲基2,5-脱水-3-脱氧戊酮酸酯
甲基(四氢呋喃-2-基甲基)砜
牛蝇畏
溴化锰(II)双(四氢呋喃)
溴化亚铁(II),双(四氢呋喃)
氧化芳樟醇
氘代四氢呋喃
异硫氰酸氢糠酯
异丙基-(四氢-呋喃-2-甲基)-胺
失水山梨醇
四氯双(四氢呋喃)合铌(IV)
四氢糠醇乙酸酯
四氢糠醇丙酸酯
四氢糠醇
四氢糠基硫醇
四氢呋喃氯化钛
四氢呋喃-D4
四氢呋喃-3-甲醛
四氢呋喃-2-甲醛
四氢呋喃-2-甲酰肼盐酸盐
四氢呋喃-2-乙酸
四氢呋喃
四氢-alpha-戊基-2-呋喃乙醇乙酸酯
四氢-alpha-[2-(四氢呋喃-2-基)乙基]-2-呋喃-1-丙醇
四氢-alpha,alpha,5-三甲基-5-乙烯基糠基乙酸酯
四氢-alpha,alpha,5-三甲基-5-(4-甲基-3-环己烯-1-基)呋喃-2-甲醇
四氢-N-[(四氢-2-呋喃基)甲基]-2-呋喃甲胺
四氢-N,2-二甲基-2-糠基胺
四氢-Alpha-戊基-2-呋喃甲醇乙酸酯
四氢-5-羟基呋喃-2-甲醇
四氢-5-甲基呋喃-2-甲醇
四氢-2-辛基呋喃
四氢-2-甲基-2-呋喃醇
四氢-2-呋喃基甲基3-氯丙酸酯
四氢-2-呋喃基氯乙酸甲酯
四氢-2-呋喃丙醇
四氢-2-呋喃-1-丙醇丙酸酯
呋喃,2-(二氯甲基)四氢-