2,3-二氯四氢呋喃 | 3511-19-1
中文名称
2,3-二氯四氢呋喃
中文别名
四氢-2,3-二氯呋喃;二氯四氢呋喃
英文名称
2,3-dichlorotetrahydrofuran
英文别名
2,3-Dichlor-tetrahydro-furan;2,3-dichlorooxolane
CAS
3511-19-1
化学式
C4H6Cl2O
mdl
MFCD00059736
分子量
140.997
InChiKey
ZQHLMWUFVRLDRK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:63 °C / 20mmHg
-
密度:1,34 g/cm3
-
稳定性/保质期:
在常温常压下,该物质是稳定的。
计算性质
-
辛醇/水分配系数(LogP):1.6
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:9.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
安全说明:S26,S36/37/39
-
危险类别码:R36/37/38
-
RTECS号:LU0525000
-
海关编码:2932190090
-
储存条件:常温、避光、通风干燥处,密封保存。
SDS
Section I.Chemical Product and Company Identification
Chemical Name 2,3-Dichlorotetrahydrofuran
Portland OR
Synonym Tetrahydro-2,3-dichlorofuran
Chemical Formula C4H6Cl2O
CAS Number 3511-19-1
Section II. Composition and Information on Ingredients
Chemical Name CAS Number Percent (%) TLV/PEL Toxicology Data
2,3-Dichlorotetrahydrofuran 3511-19-1 Min. 95.0 Not available. Not available.
(GC)
Section III. Hazards Identification
Acute Health Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
CARCINOGENIC EFFECTS : Not available.
Chronic Health Effects
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is
not known to aggravate existing medical conditions.
Section IV. First Aid Measures
Eye Contact Check for and remove any contact lenses. IMMEDIATELY flush eyes with runing water for at least 15 minutes. keeping
eyelids open. COLD water may be used. DO NOT use an eye oitment. Flush eyes with running water for a minimum of
15 minutes, occasionally lifting the upper eyelids. Seek medical attention. Treat symptomatically and supportively.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thorough wash the contaminated skin with
running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin. COLD water
may be used. Cover the irritated skin with an emollient. Seek medical attention. Treat symptomatically and supportively.
Wash any contaminated clothing before reusing.
Inhalation If the victim is not breathing, perform artificial respiration. Loosen tight clothing such as a collar, tie, belt or waistband. If
breathing is difficult, oxygen can be administered. Seek medical attention. Treat symptomatically and supportively.
INDUCE VOMITING by sticking finger in throat. Lower the head so that the vomit will not reenter the mouth and throat.
Ingestion
Loosen tight clothing such as a collar, tie, belt, or waistband. If the victim is not breathing, administer artificial respiration.
Examine the lips and mouth to ascertain whether the tissues are damaged, a possible indication that the toxic material
was ingested; the absence of such signs, however, is not conclusive. Seek immediate medical attention and, if possible,
show the chemical label. Treat symptomatically and supportively.
Section V. Fire and Explosion Data
Not available.
Flammability May be combustible at high temperature. Auto-Ignition
Flammable Limits
Flash Points Not available.
Not available.
Combustion Products These products are toxic carbon oxides (CO, CO 2), halogenated compounds.
WARNING: Highly toxic HCl gas is produced during combustion.
Fire Hazards
No specific information is available regarding the flammability of this compound in the presence of various materials.
Explosion Hazards Risks of explosion of the product in presence of mechanical impact: Not available.
Risks of explosion of the product in presence of static discharge: Not available.
No additional information is available regarding the risks of explosion.
Fire Fighting Media
SMALL FIRE: Use DRY chemicals, CO 2, water spray or foam.
and Instructions LARGE FIRE: Use water spray, fog or foam. DO NOT use water jet.
Continued on Next Page
2,3-Dichlorotetrahydrofuran
Section VI. Accidental Release Measures
Spill Cleanup In case of a spill and/or a leak, always shut off any sources of ignition, ventilate the area, and exercise caution. Absorb
Instructions with an inert material and put the spilled material in an appropriate waste disposal. Finish cleaning the spill by rinsing any
contaminated surfaces with copious amounts of water. Consult federal, state, and/or local authorities for assistance on
disposal.
Section VII. Handling and Storage
Handling and Storage Keep away from heat and sources of ignition. Mechanical exhaust required. When not in use, tightly seal the container
and store in a dry, cool place. Avoid excessive heat and light. Do not breathe gas, fumes, vapor or spray.
Information
Always store away from incompatible compounds such as oxidizing agents.
Section VIII. Exposure Controls/Personal Protection
Engineering Controls Provide exhaust ventilation or other engineering controls to keep the airborne concentrations of vapors below their
respective threshold limit value. Ensure that eyewash station and safety shower is proximal to the work-station location.
Personal Protection Splash goggles. Lab coat. Vapor and dust respirator. Gloves. Suggested protective clothing might not be sufficient;
consult a specialist BEFORE handling this product.
Exposure Limits Not available.
Section IX. Physical and Chemical Properties
Solubility
Physical state @ 20°C Liquid. Not available.
1.34
Specific Gravity
Molecular Weight 141 Partition Coefficient Not available.
Boiling Point 93°C @ 70mm Hg Vapor Pressure Not available.
Melting Point Not available. Vapor Density Not available.
Not available. Volatility Not available.
Refractive Index
Critical Temperature Not available. Odor Not available.
Viscosity Not available. Taste Not available.
Section X. Stability and Reactivity Data
Stability
This material is stable if stored under proper conditions. (See Section VII for instructions)
Conditions of Instability Avoid excessive heat and light.
Incompatibilities
Reactive with oxidizing agents.
Section XI. Toxicological Information
RTECS Number LU0525000
Eye contact. Ingestion. Inhalation.
Routes of Exposure
Toxicity Data Not available.
Chronic Toxic Effects CARCINOGENIC EFFECTS : Not available.
MUTAGENIC EFFECTS : Not available.
TERATOGENIC EFFECTS : Not available.
DEVELOPMENTAL TOXICITY: Not available.
There is no known effect from chronic exposure to this product. Repeated or prolonged exposure to this compound is not
known to aggravate existing medical conditions.
Acute Toxic Effects No specific information is available in our data base regarding the toxic effects of this material for humans. However,
exposure to any chemical should be kept to a minimum. Skin and eye contact may result in irritation. May be harmful if
inhaled or ingested. Always follow safe industrial hygiene practices and wear proper protective equipment when handling
this compound.
Continued on Next Page
2,3-Dichlorotetrahydrofuran
Section XII. Ecological Information
Ecotoxicity Not available.
Not available.
Environmental Fate
Section XIII. Disposal Considerations
Recycle to process, if possible. Consult your local or regional authorities. You may be able to dissolve or mix material with
Waste Disposal
a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system. Observe all
federal, state, and local regulations when disposing of the substance.
Section XIV. Transport Information
DOT Classification Not a DOT controlled material (United States).
Not applicable.
PIN Number
Proper Shipping Name
Not applicable.
Packing Group (PG) Not applicable.
DOT Pictograms
Section XV. Other Regulatory Information and Pictograms
TSCA Chemical Inventory This product is NOT on the EPA Toxic Substances Control Act (TSCA) inventory. The following notices are required by 40
CFR 720.36 (C) for those products not on the inventory list:
(EPA)
(i) These products are supplied solely for use in research and development by or under the supervision of a technically
qualified individual as defined in 40 CFR 720.0 et sec.
(ii) The health risks of these products have not been fully determined. Any information that is or becomes available will be
supplied on an MSDS sheet.
WHMIS Classification Not controlled under WHMIS (Canada).
(Canada)
EINECS Number (EEC) Not available.
EEC Risk Statements Not available.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
2,3-二氯四氢呋喃与四氢呋喃类似,是一种用途广泛的有机溶剂。它能溶解除聚乙烯、聚丙烯及氟树脂以外的几乎所有聚合物,特别适用于聚乙烯、聚偏二氯乙烯树脂和丁苯橡胶。在有机合成中,2,3-二氯四氢呋喃常作为溶剂使用。此外,该物质还大量用作粘结剂。
2,3-二氯四氢呋喃也应用于精密磁带工业,并且其聚合物四亚甲基醚二醇是合成聚氯氮酸弹性纤维和聚氨酯弹性体的原料。
制备以硫酰氯和四氢呋喃为原料,采用四氯化碳作为溶剂,合成了2,3-二氯四氢呋喃。优化后的反应条件包括:四氢呋喃1.4摩尔、硫酰氯1摩尔及四氯化碳50毫升,在65~75℃下进行一小时的反应,最终产率达到79.3%,气相色谱分析结果显示产品纯度为95.6%。通过红外光谱(IR)、氢核磁共振(HNMR)和GC-MS确认了产品的化学结构。
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,3,3-三氯四氢呋喃 (+/-)-2,3,3-Trichlorotetrahydrofuran 17557-41-4 C4H5Cl3O 175.442 3-氯四氢呋喃 3-chlorotetrahydrofuran 19311-38-7 C4H7ClO 106.552
反应信息
-
作为反应物:描述:2,3-二氯四氢呋喃 在 sodium tetrahydroborate 作用下, 以 二乙二醇二甲醚 为溶剂, 反应 5.0h, 以93%的产率得到3-氯四氢呋喃参考文献:名称:一种3-卤代四氢呋喃的制备方法摘要:本发明公开了一种3‑卤代四氢呋喃的制备方法。所述方法包括以下步骤:在有机溶剂中,0‑85℃温度下,将式I所示的化合物与还原剂反应,即可;其中,R1和R2相同,均选自氯或溴;所述的还原剂为硼氢化钠或硼氢化钾;所述的有机溶剂为乙二醇二甲醚、2‑氯乙基甲基醚、乙醚、四氢呋喃或甲基四氢呋喃。本发明所述的方法反应步骤少、工艺简单,产品收率可达80%‑95%,且原料成本低,设备投入少,产品价格有优势;此外所述方法污染小,对环境友好,适合大规模工业化生产。公开号:CN109748892B
-
作为产物:描述:参考文献:名称:Crombie, Leslie; Rainbow, Linda J., Journal of the Chemical Society. Perkin transactions I, 1994, # 6, p. 673 - 688摘要:DOI:
文献信息
-
Photochemical and Photophysical Behavior of<i>p</i>-Methoxyphenyl alkenyl Phenanthrenecarboxylates. I. Structure and Competitive Formation of Intramolecular Cycloaddition Products作者:Hirochika Sakuragi、Katsumi Tokumaru、Hiroki Itoh、Kohki Terakawa、Katsuo Kikuchi、R. A. Caldwell、Chien-Chung HsuDOI:10.1246/bcsj.63.1049日期:1990.4entenyl, (E)-4-(p-methoxyphenyl)-4-hexenyl, and (E)-5-(p-methoxyphenyl)-5-heptenyl 9-phenanthrenecarboxylates (B-2E, B-3E, and B-4E, respectively) in benzene gave intramolecular [2+2] cycloadducts, cyclobutane derivatives (CB-2, CB-3, and CB-4, respectively) possessing the same conformation as an intermolecular [2+2] cycloadduct (CB-0) between methyl 9-phenanthrenecarboxylate (9-MCP) and trans-anethole(E)-3-(对甲氧基苯基)-3-戊烯基、(E)-4-(对甲氧基苯基)-4-己烯基和(E)-5-(对甲氧基苯基)-5-庚烯基的辐照苯中的 9-菲羧酸盐(分别为 B-2E、B-3E 和 B-4E)产生分子内 [2+2] 环加合物,环丁烷衍生物(分别为 CB-2、CB-3 和 CB-4)具有与 9-菲羧酸甲酯 (9-MCP) 和反式茴香脑 (t-AN) 以及氧杂环丁烷衍生物 (OX-2、OX-3 和 OX) 之间的分子间 [2+2] 环加合物 (CB-0) 的构象相同-4,分别)由羰基和烯烃双键之间的分子内环加成产生。然而,(E)-4-(对甲氧基苯基)-3-丁烯基和(E)-5-(对甲氧基苯基)-4-戊烯基9-菲羧酸酯(分别为A-2E和A-3E)仅提供产物衍生自氧杂环丁烷前体。
-
[EN] SUBSTITUTED ADENINES AND THE USE THEREOF<br/>[FR] ADENINES SUBSTITUEES ET LEUR UTILISATION申请人:ASTRAZENECA AB公开号:WO2006040558A1公开(公告)日:2006-04-20This invention relates to compounds of Formula (I): and their use in the treatment of bacterial infections.这项发明涉及式(I)的化合物及其在治疗细菌感染中的应用。
-
Inhibitors of 11-beta-hydroxy steroid dehydrogenase type 1申请人:——公开号:US20030130318A1公开(公告)日:2003-07-10The present invention relates to compounds with the formula (I) 1 and also to pharmaceutical compositions comprising the compounds, as well as to the use of the compounds in medicine and for the preparation of a medicament which acts on the human 11-&bgr;-hydroxysteroid dehydrogenase type 1 enzyme.
-
3-Methylcyclohex-2-enone derivatives as initiators of cyclisation. Part 1. Introduction and synthesis of 2-substituted 3-methylcyclohex-2-enones作者:Joseph A. Amupitan、Enamul Huq、Michael Mellor、Edward G. Scovell、James K. SutherlandDOI:10.1039/p19830000747日期:——A series of C-2 substituted 3-methylcyclohex-2-enones has been prepared using the Hagemann ester route. A new synthesis of these compounds has been developed which involves the alkylation of the N,N-diethylaminoethyl ether of 1-hydroxy-5-methylcyclohexa-1,5-diene. The cyclohexenones have been converted into the corresponding α,β-epoxyketones using alkaline hydrogen peroxide.
-
Studies on antitumor agents. IV. Syntheses and antitumor activities of compounds related to 1-(tetrahydro-2-furanyl)-5-fluorouracil metabolites.作者:SHUICHI UEDA、SETSUO TAKEDA、ICHIRO YAMAWAKI、JUNICHI YAMASHITA、MITSUGI YASUMOTO、SADAO HASHIMOTODOI:10.1248/cpb.30.125日期:——A hydroxylated metabolite of 1-(tetrahydro-2-furanyl)-5-fluorouracil (FT), 1-(trans-3-hydroxytetrahydro-2-furanyl)-5-fluorouracil (trans-3'-OH-FT, VIII) and its isomer, 1-(cis-3-hydroxytetrahydro-2-furanyl)-5-fluorouracil (cis-3'-OH-FT, VI), were synthesized and isolated at high purity. As compounds related to FT metabolites, 2, 3'-anhydro-1-(cis-3-hydroxytetrahydro-2-furanyl)-5-fluorouracil (2, 3'-anhydro-FT, V), 1-(2, 5-dihydro-2-furanyl)-5-fluorouracil (3', 4'-dehydro-FT, XII) and 1-(5-acetoxytetrahydro-2-furanyl)-5-fluorouracil (5'-AcO-FT, XI) were also synthesized. The antitumor activities of these compounds against sarcoma 180 and L 1210 were examined. The activities of cis-3'-OH-FT (VI) and 2, 3'-anhydro-FT (V) were found to be lower than that of FT. The activity of 5'-AcO-FT (XI) was the same as that of FT. 3', 4'-Dehydro-FT (XII) showed much greater activity than FT.合成并分离出了 1-(四氢-2-呋喃基)-5-氟尿嘧啶(FT)的羟基代谢产物 1-(反式-3-羟基四氢-2-呋喃基)-5-氟尿嘧啶(反式-3'-OH-FT,VIII)及其异构体 1-(顺式-3-羟基四氢-2-呋喃基)-5-氟尿嘧啶(顺式-3'-OH-FT,VI),其纯度很高。作为 FT 代谢物的相关化合物,2, 3'-脱水-1-(顺式-3-羟基四氢-2-呋喃基)-5-氟尿嘧啶(2, 3'-脱水-FT,V)、1-(2、还合成了 1-(2,5-二氢-2-呋喃基)-5-氟尿嘧啶(3',4'-脱氢-FT,XII)和 1-(5-乙酰氧基四氢-2-呋喃基)-5-氟尿嘧啶(5'-AcO-FT,XI)。研究了这些化合物对肉瘤 180 和 L 1210 的抗肿瘤活性。发现顺式-3'-OH-FT(VI)和 2,3'-脱水-FT(V)的活性低于 FT。5'-AcO-FT (XI) 的活性与 FT 相同。3',4'-脱氢-FT(XII)的活性远高于 FT。
表征谱图
-
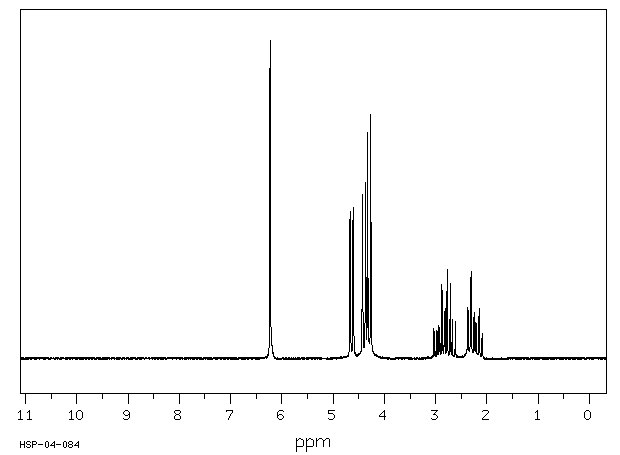
氢谱1HNMR
-
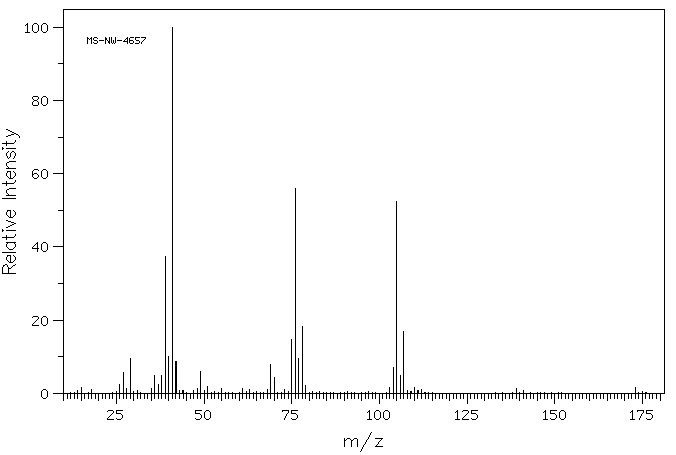
质谱MS
-
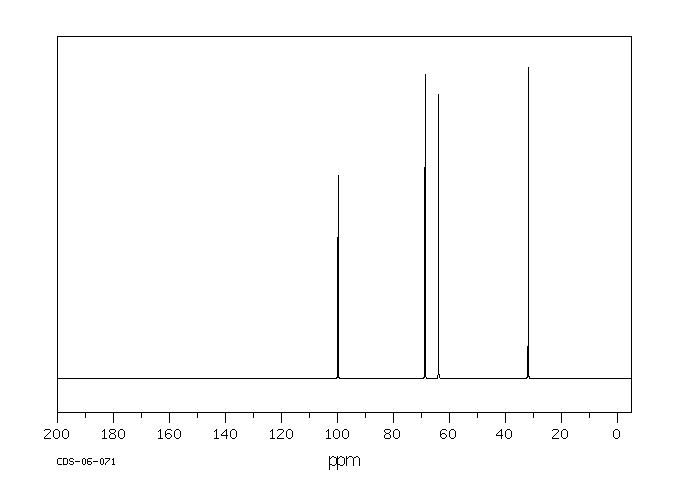
碳谱13CNMR
-
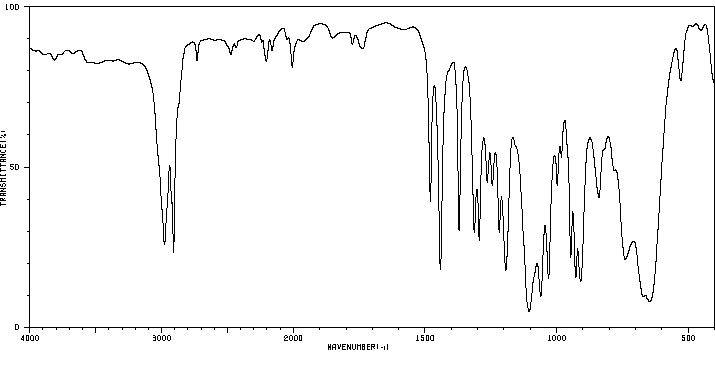
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
顺式-环氧丙烷-3,4-二胺二盐酸盐
顺式-2-(碘甲基)-3-羟基四氢呋喃
顺式-2-(碘甲基)-3-羟基四氢呋喃
青榄呋喃
茶香螺烷
苯胺,4-(2-哌嗪基)-
碳氯灵
硼烷四氢呋喃络合物
硫丹乙酯
甲基甲丙烯酰酸酯-叔丁基甲丙烯酰酸酯-月桂基甲丙烯酰酸酯共聚物
甲基丙烯酸四氢糠基酯
甲基[(噁戊环-2-基)甲基]胺盐酸
甲基2,5-脱水-3-脱氧戊酮酸酯
甲基(四氢呋喃-2-基甲基)砜
牛蝇畏
溴化锰(II)双(四氢呋喃)
溴化亚铁(II),双(四氢呋喃)
氧化芳樟醇
氘代四氢呋喃
异硫氰酸氢糠酯
异丙基-(四氢-呋喃-2-甲基)-胺
失水山梨醇
四氯双(四氢呋喃)合铌(IV)
四氢糠醇乙酸酯
四氢糠醇丙酸酯
四氢糠醇
四氢糠基硫醇
四氢呋喃氯化钛
四氢呋喃-D4
四氢呋喃-3-甲醛
四氢呋喃-2-甲醛
四氢呋喃-2-甲酰肼盐酸盐
四氢呋喃-2-乙酸
四氢呋喃
四氢-alpha-戊基-2-呋喃乙醇乙酸酯
四氢-alpha-[2-(四氢呋喃-2-基)乙基]-2-呋喃-1-丙醇
四氢-alpha,alpha,5-三甲基-5-乙烯基糠基乙酸酯
四氢-alpha,alpha,5-三甲基-5-(4-甲基-3-环己烯-1-基)呋喃-2-甲醇
四氢-N-[(四氢-2-呋喃基)甲基]-2-呋喃甲胺
四氢-N,2-二甲基-2-糠基胺
四氢-Alpha-戊基-2-呋喃甲醇乙酸酯
四氢-5-羟基呋喃-2-甲醇
四氢-5-甲基呋喃-2-甲醇
四氢-2-辛基呋喃
四氢-2-甲基-2-呋喃醇
四氢-2-呋喃基甲基3-氯丙酸酯
四氢-2-呋喃基氯乙酸甲酯
四氢-2-呋喃丙醇
四氢-2-呋喃-1-丙醇丙酸酯
呋喃,2-(二氯甲基)四氢-