沙利度胺 | 50-35-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:269-271°C
-
沸点:401.48°C (rough estimate)
-
密度:1.2944 (rough estimate)
-
溶解度:在45%(w/v)aq2-羟丙基-β-环糊精溶解度为:0.6 mg/mL
-
最大波长(λmax):300nm(lit.)
-
物理描述:Thalidomide appears as needles or white powder. (NTP, 1992)
-
颜色/状态:Needles
-
蒸汽压力:2.05X10-14 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
分解:When heated to decomposition it emits toxic fumes of /nitrogen oxides/.
-
解离常数:pKa1 = 11.59 (secondary amine); pKa2 = 16.74 (imide) (est)
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:19
-
可旋转键数:1
-
环数:3.0
-
sp3杂化的碳原子比例:0.23
-
拓扑面积:83.6
-
氢给体数:1
-
氢受体数:4
ADMET
安全信息
-
危险等级:6.1(b)
-
危险品标志:T
-
安全说明:S22,S26,S36/37/39,S45,S53
-
危险类别码:R61,R21,R22,R62,R25,R46
-
WGK Germany:3
-
海关编码:2925190090
-
危险品运输编号:UN 2811 6.1/PG 3
-
危险类别:6.1(b)
-
RTECS号:TI4375000
-
包装等级:III
-
危险标志:GHS07,GHS08
-
危险性描述:H302,H360D
-
危险性防范说明:P201,P308 + P313
-
储存条件:本品应密封避光并存放在干燥处保存。
SDS
模块 1. 化学品
产品名称: (±)-Thalidomide
5.3
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
急性毒性(经皮) 第4级
生殖毒性 第2级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽会中毒。
皮肤接触有害
怀疑会损害生育能力或胎儿
防范说明
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 食入:立即呼叫解毒中心/医生。
皮肤接触:用大量肥皂和水轻轻洗。
被污染的衣物清洗后方可重新使用。
如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
(+/-)-沙利度胺
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): (+/-)-沙利度胺
百分比: >98.0%(HPLC)(N)
CAS编码: 50-35-1
俗名: N-(2,6-Dioxo-3-piperidinyl)phthalimide
分子式: C13H10N2O4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
(+/-)-沙利度胺
模块 8. 接触控制和个体防护
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色-极淡的黄色
气味: 无资料
pH: 无数据资料
熔点: 276°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 不溶于(60mg/L, 25°C)
[其他溶剂]
易溶于: 二甲基甲酰胺, 吡啶, 二氧六环
极微溶于: 甲醇, 丙酮, 乙醇, 乙酸乙酯, 冰乙酸
不溶于: 醚, 苯, 氯仿
log水分配系数 = 3.09
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: orl-rat LD50:113 mg/kg
skn-rat LD50:1550 mg/kg
ipr-rat LD50:>6 g/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: cyt-hmn-lym 1 mg/L
mmo-omi 10 g/L (-S9)
致癌性: scu-mus TDLo:34 g/kg/57W-I
IARC = 无资料
NTP = 无资料
生殖毒性: orl-wmn TDLo:16 mg/kg(28-35D preg)
orl-rat TDLo:28 g/kg(3D male/3D pre-22D preg)
ipr-rat TDLo:1200 mg/kg(9-11D preg)
ivn-rat TDLo:45 mg/kg(12D preg)
RTECS 号码: TI4375000
模块 12. 生态学信息
生态毒性:
(+/-)-沙利度胺
模块 12. 生态学信息
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 3.09
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第1项 毒害品。
UN编号: 2811
正式运输名称: 有毒固体, 有机物, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
沙利度胺(Thalidomide),又称反应停、酞胺哌酮、酞咪哌啶酮等,是一种合成的谷氨酸衍生物。在室温下它呈现为白色结晶性粉末,无味且微溶于水或甲醇,易溶于二甲基甲酰胺或吡啶中,但不溶解于乙醚、氯仿或苯。
20世纪50年代初期,沙利度胺最初被开发用于治疗癫痫,但由于疗效不佳,不久之后它转而作为睡眠辅助药物,并在怀孕期间广泛用于缓解孕妇的恶心症状。然而,在20世纪60年代初,沙利度胺引起了一系列严重的副作用事件——即所谓的反应停事件。大量婴儿因服用了这种药物而导致畸形(例如:短肢畸形、长骨缺损、耳廓缺失、心脏和胃肠道畸形等)。这一系列事件促使许多国家禁止了该药的使用,并将其撤出市场。
尽管如此,科学家们并未放弃对沙利度胺的研究。它在免疫调节、抗炎以及抗血管生成方面展现出令人惊讶的效果,尤其在治疗某些癌症症状上显示出潜力。近年来,沙利度胺被重新用于研究治疗多种疾病的潜在价值。
合成方法 制备粗品将10公斤N-邻苯二甲酸谷氨酸酐熔化于反应釜中,并通入氨气进行反应。完成反应后,将溶液倒入水中搅拌并冷却,通过过滤分离产物,再用洗涤水清洁滤饼以获得沙利度胺粗品。
制备高纯度产品称取100克(HPLC 97.3%)的粗品,加入200克二甲基亚砜以及1克活性炭。加热至100℃溶解后趁热进行抽滤处理。将所得溶液冷却至0℃并再次抽滤,用乙醇洗涤滤出物,并在80℃下干燥以得到高纯度的沙利度胺。
生物活性沙利度胺作为一种镇静剂和免疫调节剂,在多种癌症治疗中显示出潜力。研究发现它能够通过CRBN-DDB1-Cul4A复合体抑制E3泛素连接酶,并且还能选择性地降低脂多糖和其他激动剂刺激人体单核细胞产生的肿瘤坏死因子α(TNF-α)水平。
体内与体外研究 体外研究Thalidomide通过肝脏代谢形成环氧化物,成为活性致畸物质。它能抑制TNF-α的产生,并通过增强mRNA降解、诱导细胞凋亡及G1期生长停滞来直接作用于多发性骨髓瘤(MM)细胞系以及对抗melphalan, doxorubicin和dexamethasone治疗无效的患者。
此外,Thalidomide还能作为原代人T细胞在体外实验中的协同刺激分子,通过与T细胞受体复合物作用增强白细胞介素-2介导的T细胞增殖及干扰素γ生成。同时它还会增加CD4+ T细胞在无存在情况下对异种树突状细胞诱导初级CD8+细胞毒性T细胞应答的能力。
体内研究一项实验显示,注射200毫克/千克剂量的Thalidomide能够导致兔子角膜血管化的抑制,在三个独立实验中观察到的平均抑制率为36%(范围从30%-51%)。
化学性质沙利度胺具有无色、无味和脂溶性的特性,是一种白色结晶粉末。其分子式为C₁₃H₁₀N₂O₄。
用途作为镇静剂,沙利度胺对各类麻风病症状如发热、结节红斑、神经痛、关节痛及淋巴结肿大等具有显著疗效。此外,它还可以用于预防和控制麻风结节性红斑皮肤症状的复发。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(2,6-二氧代哌啶烯-3-基)-苄甲内酰胺 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione 26581-81-7 C13H12N2O3 244.25 2-(1-羟基-2,6-二氧代哌啶-3-基)异吲哚-1,3-二酮 N-Hydroxythalidomide 126663-38-5 C13H10N2O5 274.233 N-(4-甲氧基苄基)沙利度胺 2-[1-(4-methoxybenzyl)-2,6-dioxopiperidine-3-yl]isoindole-1,3-dione 222713-07-7 C21H18N2O5 378.384 —— 2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline-5-carboxylic acid 1216805-11-6 C14H10N2O6 302.243 —— 2-(2,6-dioxopiperidin-3-yl)-1,3-dioxoisoindoline-4-carboxylic acid 1547163-38-1 C14H10N2O6 302.243 N-α-邻苯二酰-L-谷氨酰胺 phthalyl-L-glutamine 3343-29-1 C13H12N2O5 276.249 2-(1,3-二氧代异吲哚啉-2-基)-5-氨基-5-氧代戊酸 2-phthalimidoglutaramic acid 7607-72-9 C13H12N2O5 276.249 2-(((2,6-二氧代-3-哌啶基)氨基)羰基)-苯甲酸 N-(o-Carboxybenzoyl)-D,L-glutamic acid imide 6139-18-0 C13H12N2O5 276.249 (L)-2-苯二甲酰亚氨基戊二酸 N-phthalylglutamic acid 6349-98-0 C13H11NO6 277.233 PHT-谷氨酸 N-phthaloyl-L-glutamic acid 340-90-9 C13H11NO6 277.233 N-酞酰基-DL-谷氨酸酐 N-phthalyl-L-glutamic anhydride 3343-28-0 C13H9NO5 259.218 —— 2-(cyclopent-2-en-1-yl)isoindoline-1,3-dione 100727-30-8 C13H11NO2 213.236 —— 1-p-methoxybenzyl-3-phthalimido-5-toluenesulfonylpiperidine-2,6-dione 485817-55-8 C28H24N2O7S 532.574 —— methyl 2-phthalimidoacrylate 26878-24-0 C12H9NO4 231.208 —— ethyl α-phthalimidoacrylate 24249-89-6 C13H11NO4 245.235 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 反应停 (R)-thalidomide 2614-06-4 C13H10N2O4 258.233 (-)-沙利度胺 (S)-thalidomide 841-67-8 C13H10N2O4 258.233 1-甲基-3-(1,3-二氧代异吲哚啉-2-基)-2,6-哌啶二酮 2-(1-methyl-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione 42472-93-5 C14H12N2O4 272.26 2-(2,6-二氧代哌啶烯-3-基)-苄甲内酰胺 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione 26581-81-7 C13H12N2O3 244.25 —— 2-(1-benzyl-2,6-dioxopiperidin-3-yl)isoindol-1,3-dione 24666-53-3 C20H16N2O4 348.358 —— (R,S)-2-(2,6-dioxo-1-(pent-4-ynyl)piperidin-3-yl)isoindoline-1,3-dione 1252785-14-0 C18H16N2O4 324.336 —— (±)-2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione 24666-54-4 C15H14N2O5 302.287 —— (2-[1-(hydroxymethyl)-2,6-dioxopiperidin-3-yl]isoindoline-1,3-dione) 145945-21-7 C14H12N2O5 288.26 —— N,N-(N,N-phthaloyl-glutamoyl)-glycine 100881-15-0 C15H12N2O6 316.27 N-(4-甲氧基苄基)沙利度胺 2-[1-(4-methoxybenzyl)-2,6-dioxopiperidine-3-yl]isoindole-1,3-dione 222713-07-7 C21H18N2O5 378.384 —— 2-{1-[(methylthio)methyl]-2,6-dioxopiperidin-3-yl}-1H-isoindole-1,3(2H)-dione 1083189-78-9 C15H14N2O4S 318.353 —— (R,S)-2-(1-(azidomethyl)-2,6-dioxopiperidin-3-yl)isoindoline-1,3-dione —— C14H11N5O4 313.272 —— 2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindolin-1,3-dione bromoacetate 911315-12-3 C17H15BrN2O6 423.22 —— 2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindolin-1,3-dione (S)-2-amino-3-methylbutanoate 911315-07-6 C20H23N3O6 401.419 —— KE3 197956-35-7 C18H16N2O8 388.334 —— 1,3-dioxo-2-(1-tert-butoxycarbonyl-2,6-dioxopiperidin-3-yl)isoindoline 220460-69-5 C18H18N2O6 358.351 —— N-tert-butyloxycarbonyl-2-aminoacetic acid-[3-(1,3-dihydro-1,3-dioxo-2H-isoindole-2-yl)-2,6-dioxo-piperidine-1-yl-methyl]ester 197956-27-7 C21H23N3O8 445.429 —— 2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindolin-1,3-dione (S)-2-amino-3-phenylpropanoate 911315-23-6 C24H23N3O6 449.463 —— N-tert-butyloxycarbonyl-(aminoacetylamino)acetic acid-[3-(1,3-dihydro-1,3-dioxo-2H-isoindole-2-yl)-2,6-dioxopiperidine-1-yl-methyl]ester 197956-33-5 C23H26N4O9 502.481 —— 3'-fluorothalidomide 220460-55-9 C13H9FN2O4 276.224 —— 2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindolin-1,3-dione (S)-2-(2-bromoacetamido)-3-methylbutanoate 911315-09-8 C22H24BrN3O7 522.352 —— 3-(1-hydroxy-3-oxoisoindolin-2-yl)piperidine-2,6-dione 58585-25-4 C13H12N2O4 260.249 —— 2-(1-(2-hydroxyethyl)-2,6-dioxopiperidin-3-yl)isoindolin-1,3-dione (S)-2-(tert-butoxycarbonylamino)-3-methylbutanoate 911315-06-5 C25H31N3O8 501.536 —— (3-(1,3-dioxoisoindolin-2-yl)-2,6-dioxopiperidin-1-yl)methyl nicotinate 1246252-93-6 C20H15N3O6 393.356 —— 1-thioxo-3-oxo-2-(2-oxo-6-thioxopiperidin-3-yl)isoindoline 628337-08-6 C13H10N2O2S2 290.367 —— N-(cholesteryloxyacetyl)-thalidomide 690993-47-6 C42H56N2O6 684.916 —— N-(cholesteryloxycarbonyl)-thalidomide 690993-48-7 C41H54N2O6 670.89 —— 1,3-dioxo-2-(1-tert-butoxycarbonyl-2,6-dioxo-3-fluoropiperidin-3-yl)isoindoline 243469-07-0 C18H17FN2O6 376.341 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:[EN] COMPOUNDS MODULATING PROTEIN RECRUITMENT AND/OR DEGRADATION
[FR] COMPOSÉS MODULANT LE RECRUTEMENT ET/OU LA DÉGRADATION DE PROTÉINES摘要:这项发明提供了用于通过泛素蛋白酶体途径降解蛋白质的 cereblon 结合物,用于治疗应用。公开号:WO2021126973A1 -
作为产物:描述:参考文献:名称:A Concise Two-Step Synthesis of Thalidomide摘要:A two-step synthesis of thalidomide is presented. The sequence requires no purifications. Treatment of L-glutamine with N-carbethoxyphthalimide produces N-phthaloyl-L-glutamine. Cyclization of N-phthaloyl-L-glutamine to afford thalidomide is accomplished by treatment with CDI in the presence of a catalytic amount of DMAP.DOI:10.1021/op980201b
文献信息
-
[EN] TARGETED DELIVERY AND PRODRUG DESIGNS FOR PLATINUM-ACRIDINE ANTI-CANCER COMPOUNDS AND METHODS THEREOF<br/>[FR] ADMINISTRATION CIBLÉE ET CONCEPTIONS DE PROMÉDICAMENTS POUR COMPOSÉS ANTICANCÉREUX À BASE DE PLATINE ET D'ACRIDINE ET MÉTHODES ASSOCIÉES申请人:WAKE FOREST SCHOOL OF MEDICINE公开号:WO2013033430A1公开(公告)日:2013-03-07Acridine containing cispiaiin compounds have been disclosed that show greater efficacy against cancer than other cisplatin compounds. Methods of delivery of those more effective eisp!aiin compounds to the nucleus in cancer ceils is disclosed using one or more amino acids, one or more sugars, one or more polymeric ethers, C i^aikylene-phenyl-NH-C(0)-R.15, folic acid, av03 iniegriii RGD binding peptide, tamoxifen, endoxifen, epidermal growth factor receptor, antibody conjugates, kinase inhibitors, diazoles, triazol.es, oxazoies, erlotinib, and/or mixtures thereof; wherein R]§ is a peptide.
-
[EN] ACC INHIBITORS AND USES THEREOF<br/>[FR] INHIBITEURS DE L'ACC ET UTILISATIONS ASSOCIÉES
-
Compositions for Treatment of Cystic Fibrosis and Other Chronic Diseases申请人:Vertex Pharmaceuticals Incorporated公开号:US20150231142A1公开(公告)日:2015-08-20The present invention relates to pharmaceutical compositions comprising an inhibitor of epithelial sodium channel activity in combination with at least one ABC Transporter modulator compound of Formula A, Formula B, Formula C, or Formula D. The invention also relates to pharmaceutical formulations thereof, and to methods of using such compositions in the treatment of CFTR mediated diseases, particularly cystic fibrosis using the pharmaceutical combination compositions.
-
SULFONAMIDE, SULFAMATE, AND SULFAMOTHIOATE DERIVATIVES申请人:Wang Zhong公开号:US20120077814A1公开(公告)日:2012-03-29The disclosure provides biologically active compounds of formula (I): and pharmaceutically acceptable salts thereof, compositions containing these compounds, and methods of using these compounds in a variety applications, such as treatment of diseases or disorders associated with E1 type activating enzymes, and with Nedd8 activating enzyme (NAE) in particular.
-
[EN] COMPOUNDS AND METHODS FOR THE TREATMENT OF NEURODEGENERATIVE DISEASES<br/>[FR] COMPOSÉS ET PROCÉDÉS POUR LE TRAITEMENT DE MALADIES NEURODÉGÉNÉRATIVES申请人:TAVARES FRANCIS XAVIER公开号:WO2016168118A1公开(公告)日:2016-10-20Novel compounds of formula (II) are disclosed. Compounds of formula (II) comprise ornithine derivatives or compounds that may metabolize to ornithine. Also disclosed are methods for the treatment of neurodegenerative diseases such as Alzheimer's Disease using compounds of formula (II).
表征谱图
-
氢谱1HNMR
-
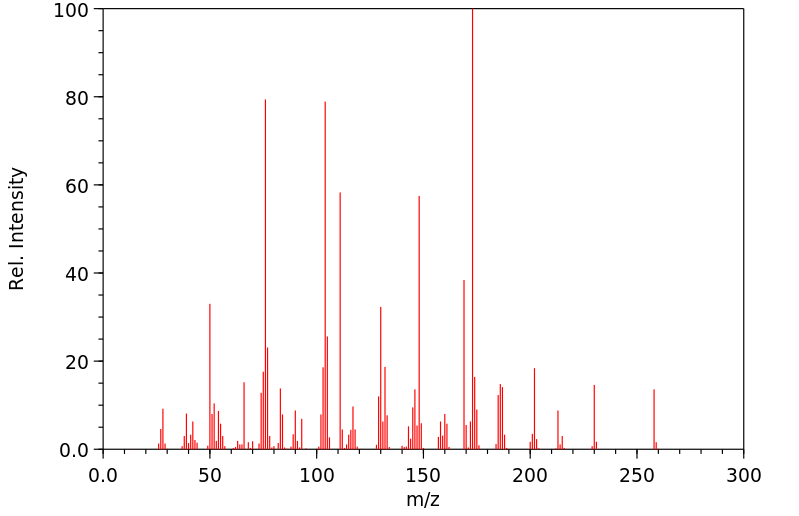
质谱MS
-
碳谱13CNMR
-
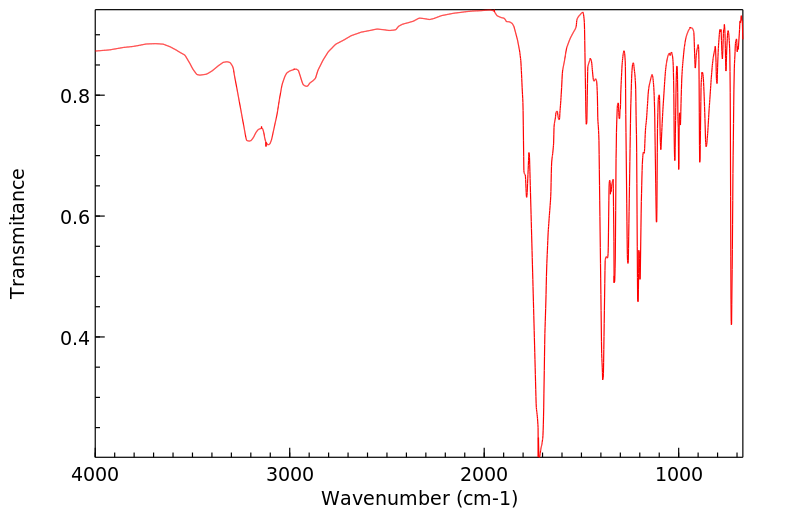
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息