(L)-2-苯二甲酰亚氨基戊二酸 | 6349-98-0
中文名称
(L)-2-苯二甲酰亚氨基戊二酸
中文别名
酞酰-DL-谷氨酸
英文名称
N-phthalylglutamic acid
英文别名
N-phthaloyl-DL-glutamic acid;2-phthalimido-glutaric acid;Phthalylglutamic acid;2-Phthalimidoglutaric acid;2-(1,3-dioxoisoindol-2-yl)pentanedioic acid
CAS
6349-98-0
化学式
C13H11NO6
mdl
——
分子量
277.233
InChiKey
FEFFSKLJNYRHQN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:189.0 to 193.0 °C
-
沸点:519.0±40.0 °C(Predicted)
-
密度:1.566±0.06 g/cm3(Predicted)
-
溶解度:在甲醇中几乎透明
计算性质
-
辛醇/水分配系数(LogP):0.4
-
重原子数:20
-
可旋转键数:5
-
环数:2.0
-
sp3杂化的碳原子比例:0.23
-
拓扑面积:112
-
氢给体数:2
-
氢受体数:6
安全信息
-
RTECS号:MA3810000
-
储存条件:室温
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 N-酞酰基-DL-谷氨酸酐 N-phthalyl-L-glutamic anhydride 3343-28-0 C13H9NO5 259.218 —— 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-4-nitro-pentanedioic acid 5-ethyl ester 1-methyl ester 204922-61-2 C16H16N2O8 364.312 —— 2-(cyclopent-2-en-1-yl)isoindoline-1,3-dione 100727-30-8 C13H11NO2 213.236 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-(3-氧代-1H-异吲哚-2-基)戊二酸 EM 138 26577-32-2 C13H13NO5 263.25 2-(1,3-二氧代异吲哚啉-2-基)-5-氨基-5-氧代戊酸 2-phthalimidoglutaramic acid 7607-72-9 C13H12N2O5 276.249 N-酞酰基-DL-谷氨酸酐 N-phthalyl-L-glutamic anhydride 3343-28-0 C13H9NO5 259.218 —— 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-4-(4-methoxy-benzylcarbamoyl)-butyric acid 912893-81-3 C21H20N2O6 396.4 —— Dimethyl (2R,4S)-4-bromo-N-phthaloylglutamate 127379-75-3 C15H14BrNO6 384.183 —— Dimethyl (2R,4R)-4-bromo-N-phthaloylglutamate —— C15H14BrNO6 384.183 —— 2-(1,3-dioxo-2,3-dihydro-1H-isoindol-2-yl)pentanedioyl dichloride 19117-51-2 C13H9Cl2NO4 314.125 沙利度胺 thalidomide 50-35-1 C13H10N2O4 258.233 —— 6-Ethyl-2-phthalimido-glutarimid 54946-21-3 C15H14N2O4 286.287 2-(2,6-二氧代哌啶烯-3-基)-苄甲内酰胺 3-(1-oxoisoindolin-2-yl)piperidine-2,6-dione 26581-81-7 C13H12N2O3 244.25 —— 2-(1-benzyl-2,6-dioxopiperidin-3-yl)isoindol-1,3-dione 24666-53-3 C20H16N2O4 348.358 N-(4-甲氧基苄基)沙利度胺 2-[1-(4-methoxybenzyl)-2,6-dioxopiperidine-3-yl]isoindole-1,3-dione 222713-07-7 C21H18N2O5 378.384 —— 1-benzyl-3-(1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione 26840-61-9 C20H18N2O3 334.375 —— 1-(4-methoxybenzyl)-3-(1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione 222713-10-2 C21H20N2O4 364.401 —— N-benzyloxythalidomide 600721-43-5 C20H16N2O5 364.357 —— 1-benzyloxy-3-(1-oxo-1,3-dihydroisoindol-2-yl)piperidine-2,6-dione 600721-44-6 C20H18N2O4 350.374 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:Synthesis and Enantiomeric Separation of 2-Phthalimidino-glutaric Acid Analogues: Potent Inhibitors of Tumor Metastasis摘要:DOI:10.1021/jm990083y
-
作为产物:描述:参考文献:名称:Process for the preparation of glutamic acid and intermediates therefor摘要:公开号:US02801250A1
文献信息
-
COMPOUNDS AND METHODS FOR THE TARGETED DEGRADATION OF ANDROGEN RECEPTOR申请人:Arvinas, Inc.公开号:US20180099940A1公开(公告)日:2018-04-12The present disclosure relates to bifunctional compounds, which find utility to degrade and (inhibit) Androgen Receptor. In particular, the present disclosure is directed to compounds, which contain on one end a cereblon ligand which binds to the E3 ubiquitin ligase and on the other end a moiety which binds Androgen Receptor, such that Androgen Receptor is placed in proximity to the ubiquitin ligase to effect degradation (and inhibition) of Androgen Receptor. The present disclosure exhibits a broad range of pharmacological activities associated with compounds according to the present disclosure, consistent with the degradation/inhibition of Androgen Receptor.
-
New synthetic routes to α-amino acids and γ-oxygenated α-amino acids. Reductive denitration and oxidative transformations of γ-nitro-α-amino acids作者:Maxwell J. Crossley、Yik M. Fung、Efstathia Kyriakopoulos、Jeffrey J. PotterDOI:10.1039/a707067e日期:——Transformation of γ-nitro-α-amino acid derivatives into α-amino acids by reductive denitration, into the γ-oxo-α-amino acids by ozonolysis of the corresponding amino acid ester nitronate derivatives, and into γ-hydroxy-α-amino acid derivatives by subsequent reduction of the oxo functionality, can be achieved in good yields. As the γ-nitro-α-amino acid derivatives are prepared from N,O-protected dehydroalanines derivable from the corresponding alanine, serine and cysteine derivatives by specific routes, the overall procedures provide a means for selective conversion of these simple α-amino acids into more complex ones.
-
A novel strategy for efficient chemoenzymatic synthesis of D-glutamine using recombinant Escherichia coli cells作者:Qinglin Du、Xiangyang Zhang、Xinru Pan、Hongjuan Zhang、Yu-Shun Yang、Junzhong Liu、Qingcai JiaoDOI:10.1016/j.molstruc.2020.128600日期:2020.11the synthesis of D-glutamine is very valuable both in academic career and potential applications. Herein, we developed a novel efficient chemoenzymatic strategy for producing D-glutamine. Initially, DL-glutamine was chemically prepared with cheap and accessible DL-glutamic acid as raw material. Subsequently, the L-glutamine among the racemic mixture was selectively hydrolyzed to L-glutamic acid by Escherichia摘要 D-谷氨酰胺是谷氨酰胺的D型立体异构体,参与多种代谢过程。寻求低成本和工业可扩展的 D-谷氨酰胺合成方法在学术生涯和潜在应用中都非常有价值。在此,我们开发了一种用于生产 D-谷氨酰胺的新型高效化学酶促策略。最初,DL-谷氨酰胺是以廉价易得的DL-谷氨酸为原料化学制备的。随后,外消旋混合物中的L-谷氨酰胺通过表达来自Ochrobactrum anthropi的L-氨基肽酶D-Ala-酯酶/酰胺酶(DmpA)的大肠杆菌全细胞系统选择性水解为L-谷氨酸。左 D-谷氨酰胺通过等电点沉淀获得,理论产率为 70%。此外,我们优化了酶解条件,以确定最佳参数为 pH 8、30 °C、0.1% (v/v) Triton X-100 和 1 mM Mn2+。这些结果表明我们的策略可能可用于工业生产中 D-谷氨酰胺的合成。
-
Facile Synthesis of Thalidomide作者:Binh Duong Vu、Ngoc Minh Ho Ba、Dinh Chau PhanDOI:10.1021/acs.oprd.9b00122日期:2019.7.19We report a simple and facile procedure for the preparation of thalidomide in two steps with a high overall yield (56%). The preparation was composed of a reaction between anhydride phthalic and l-glutamic acid to form N-phthaloyl-dl-glutamic acid (IV), and a cyclization step using IV reacted with ammonium acetate in diphenyl ether to create thalidomide. Reaction parameters reaction time, temperature
-
一种沙利度胺的合成方法
表征谱图
-
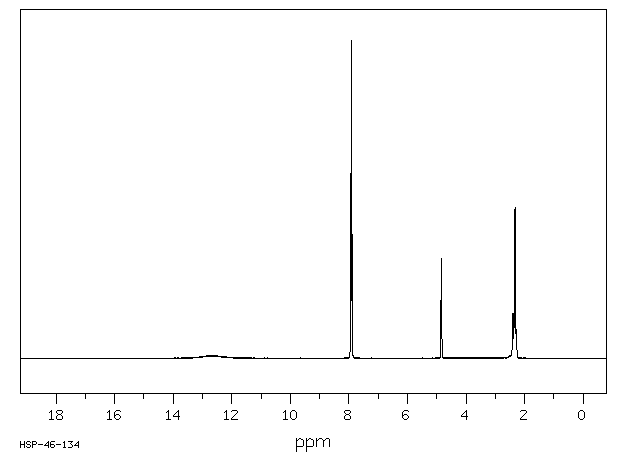
氢谱1HNMR
-
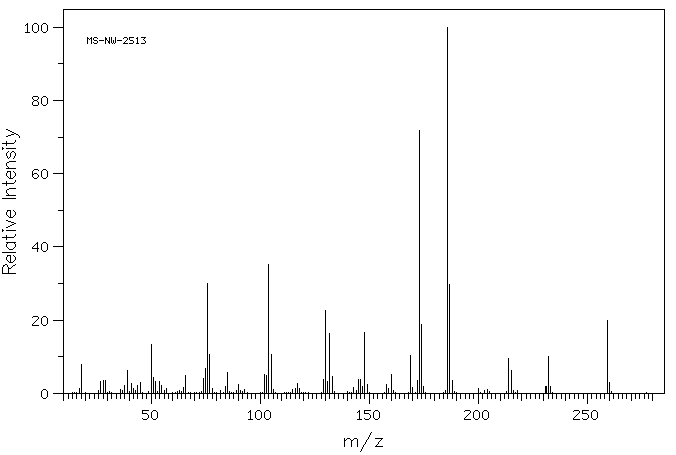
质谱MS
-
碳谱13CNMR
-
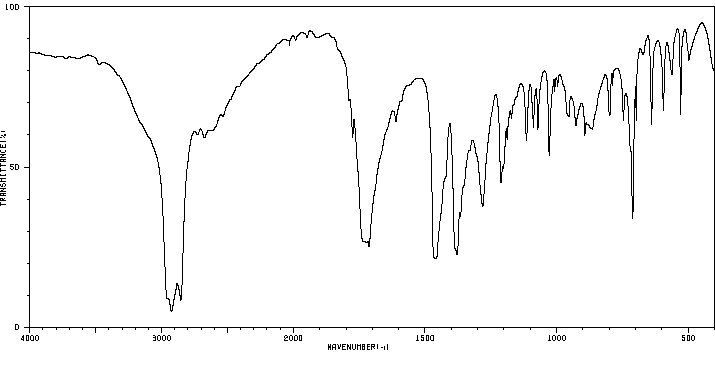
红外IR
-
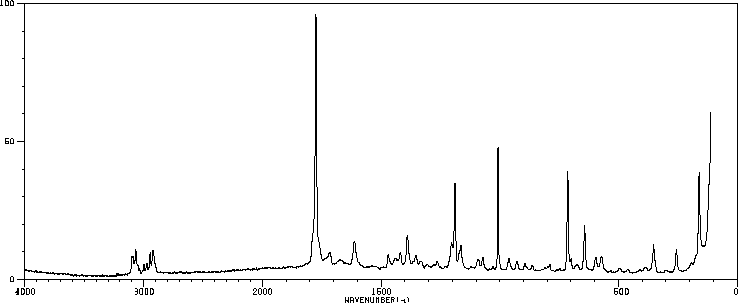
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸