溴丙酮 | 598-31-2
中文名称
溴丙酮
中文别名
1-溴-2-丙酮;1-溴丙酮;溴-2-丙酮; 1-溴丙酮; 1-溴-2-丙酮
英文名称
1-bromoacetone
英文别名
Bromoacetone;1-bromopropan-2-one;bromopropanone;1‐bromoacetone
CAS
598-31-2
化学式
C3H5BrO
mdl
MFCD00039188
分子量
136.976
InChiKey
VQFAIAKCILWQPZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-36,5°C
-
沸点:137°C
-
密度:1,63 g/cm3
-
闪点:51℃
-
物理描述:Bromoacetone appears as a clear colorless liquid turning violet on standing, even in the absence of air, and decomposing to a black resinous mass on long standing. Denser than water and poorly soluble in water. Hence sinks in water. A violent lachrymator--low concentrations are very irritating to the eyes; high concentrations or prolonged exposure at lower concentrations may have adverse health effects. Very toxic by inhalation. Contact with the liquid causes painful burns. Used as a chemical war gas.
-
颜色/状态:Colorless liquid; rapidly becomes violet even in absence of air
-
气味:PUNGENT ODOR
-
溶解度:Soluble in ethanol, ether, and acetone
-
蒸汽密度:4.75 (Air = 1)
-
蒸汽压力:9 mm Hg at 20 °C
-
稳定性/保质期:
-
分解:When heated to decomposition it emits toxic fumes of /hydrogen bromide/.
-
折光率:Index of refraction: 1.4697 at 15 °C/D
-
解离常数:pKa = 16.1
-
保留指数:751.1;751
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:5
-
可旋转键数:1
-
环数:0.0
-
sp3杂化的碳原子比例:0.666
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
ADMET
代谢
在这项研究中,考察了影响1-溴丙烷(1-BrP)处置和生物转化的因素,对象是吸入暴露(800 ppm)或静脉注射(5、20和100 mg/kg)后的雄性F344大鼠和B6C3F1小鼠。使用了(1,2,3-13C)1-BrP和(1-14C)1-BrP,通过核磁共振光谱、液相色谱-串联质谱(LC-MS/MS)和高效液相色谱耦合放射性色谱法来表征尿液中的代谢物。呼出的14CO2与释放的总溴化物的摩尔比随着剂量的增加而降低,表明在大鼠中,相对于依赖谷胱甘肽结合的途径,通过氧化代谢的1-BrP比例与剂量成反比。在静脉注射5至100 mg/kg (14C)1-BrP的大鼠和小鼠的尿液中回收到了(14C)1-BrP的等价物(大鼠13-17%,小鼠14-23%),在粪便中(< 2%)或在组织和尸体中保留(< 6%)。在大鼠和小鼠尿液中的代谢物包括N-乙酰-S-丙基半胱氨酸、N-乙酰-3-(丙硫基亚硝基)丙氨酸、N-乙酰-S-(2-羟基丙基)半胱氨酸、1-溴-2-羟基丙烷-O-葡萄糖苷酸、N-乙酰-S-(2-氧代丙基)半胱氨酸和N-乙酰-3-[(2-氧代丙基)硫基亚硝基]丙氨酸。这些代谢物可能是在1-溴丙烷氧化成1-溴-2-丙醇和溴乙酮之后,以及随后与这两种化合物之一进行谷胱甘肽结合形成的。
... In this study, the factors influencing the disposition and biotransformation of 1-bromopropane (1-BrP) were examined in male F344 rats and B6C3F1 mice following inhalation exposure (800 ppm) or intravenous administration (5, 20, and 100 mg/kg). (1,2,3-13C)1-BrP and (1-14C)1-BrP were administered to enable characterization of urinary metabolites using NMR spectroscopy, LC-MS/MS, and HPLC coupled radiochromatography. ... The molar ratio of exhaled 14CO2 to total released bromide, which decreased as dose increased, demonstrated that the proportion of 1-BrP metabolized via oxidation relative to pathways dependent on glutathione conjugation is inversely proportional to dose in the rat. (14C)1-BrP equivalents were recovered in urine (13-17%, rats; 14-23% mice), feces (< 2%), or retained in the tissues and carcass (< 6%) of rats and mice administered iv 5 to 100 mg/kg (14C)1-BrP. Metabolites characterized in urine of rats and mice include N-acetyl-S-propylcysteine, N-acetyl-3-(propylsulfinyl)alanine, N-acetyl-S-(2-hydroxypropyl)cysteine, 1-bromo-2-hydroxypropane-O-glucuronide, N-acetyl-S-(2-oxopropyl)cysteine, and N-acetyl-3-[(2-oxopropyl)sulfinyl]alanine. These metabolites may be formed following oxidation of 1-bromopropane to 1-bromo-2-propanol and bromoacetone and following subsequent glutathione conjugation with either of these compounds ...
来源:Hazardous Substances Data Bank (HSDB)
代谢
Bromoacetone is a metabolic byproduct of bromopropane. It can react with glutathione and generate conjugates that are subsequently secreted in the urine.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
有机溴化合物,如溴代丙酮,是强烈的烷基化剂。因此,它们能够轻易地修改自由的硫醇(半胱氨酸)和蛋白质表面的蛋氨酸残基,从而导致酶、转运蛋白或膜功能的破坏。最可能的蛋白质靶标之一是TRPA1离子通道,它在眼睛、鼻子、嘴巴和肺部的三叉神经感觉神经中表达。
Organobromide compounds such as bromoacetone are strong alkylating agents. Consequently they can readily modify free thiols (cysteines) and methionine residues of the surfaces of proteins leading to the disruption of enzyme, transporter or membrane functions. One of the most probable protein targets is the TRPA1 ion channel that is expressed in sensory nerves (trigeminal nerve) of the eyes, nose, mouth and lungs.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
无致癌性迹象(未被国际癌症研究机构列名)。
No indication of carcinogenicity (not listed by IARC). (L135)
来源:Toxin and Toxin Target Database (T3DB)
毒理性
一种强烈的催泪剂。吸入、摄入或皮肤接触该物质可能导致严重伤害或死亡。动物研究表明,在非常高的浓度下,它可能造成肾脏和肝脏损伤。溴代丙酮在0.1 ppm浓度下能使30%的人眼泪腺受到刺激,在1.0 ppm浓度下能使100%的人眼泪腺受到刺激。
A strong lachrymator. Inhalation, ingestion or skin contact with material may cause severe injury or death. Animal studies indicate that at very high concentrations it can cause kidney and liver damage. Bromoacetone causes ocular irritation in 30% of human subjects at 0.1 ppm and 100% of human subjects at 1.0 ppm.
来源:Toxin and Toxin Target Database (T3DB)
毒理性
该物质可以通过吸入其蒸气和摄入的方式被身体吸收。
The substance can be absorbed into the body by inhalation of its vapour and by ingestion.
来源:ILO-WHO International Chemical Safety Cards (ICSCs)
毒理性
口服(L626);吸入(L626);皮肤给药(L626)
Oral (L626) ; inhalation (L626) ; dermal (L626)
来源:Toxin and Toxin Target Database (T3DB)
安全信息
-
危险等级:6.1(a)
-
安全说明:S26,S36/37/39
-
危险类别码:R11
-
海关编码:2914700090
-
危险品运输编号:1569
-
危险类别:6.1(a)
-
包装等级:II
-
储存条件:储存注意事项: - 储存在阴凉、通风良好的库房中。 - 远离火源和热源,库温不超过35℃,相对湿度不超过85%。 - 保持容器密封。 - 应与氧化剂和食用化学品分开存放,切忌混储。 - 使用防爆型照明和通风设施。 - 禁止使用能产生火花的机械设备和工具。 - 储区应配备泄漏应急处理设备和合适的收容材料。
制备方法与用途
溴丙酮
应用 溴丙酮是有机化学中常用的丙酮化和合成杂环化合物的试剂,常用于双官能团或多官能团底物的关环反应。例如,在氢氧化钠水溶液(2.0 g/25 mL)中加入溴(0.85 mL,16.6 mmol),生成羟基丙酮;而与含羟基(或巯基)化合物如酚、醇等作用时,则可生成β-羰基醚。比如,醇钠与溴代丙酮的反应产物即为一例。
合成方法 在0°C下,向氢氧化钠溶液(2.0 g/25 mL)中缓慢加入溴(0.85 mL,16.6 mmol),搅拌15分钟。随后滴加α-氯酮溶液和乙酸的次溴酸钠溶液(4 mL+1.70 mL,1.13 mmol,0.664 M),并保持在0°C下反应1小时。通过薄层色谱监测反应进程。将反应混合物倒入饱和碳酸氢钠中,并用二氯甲烷稀释,分出有机相两次。合并有机层后加入MgSO4干燥,浓缩后再经硅胶(己烷/乙醚7/3)快速色谱纯化,最终得到溴丙酮。
化学性质 无色刺激性液体,熔点-36.5℃,沸点137℃,相对密度1.634(23℃),折光率1.4679(25℃)。易溶于乙醇和丙酮,不溶于水。
用途 有机合成中间体。
类别 有毒物品
毒性分级 高毒
急性毒性 吸入-人LCL0: 572 PPM/10分钟
可燃性危险特性 明火下可燃;受热分解产生有毒的溴化物气体
储运特性 应存放在通风低温干燥的地方,与氧化剂和食品添加剂分开存放。
灭火剂 泡沫、干粉、砂土或二氧化碳。
上下游信息
反应信息
-
作为反应物:参考文献:名称:Alcudia,F. et al., Canadian Journal of Chemistry, 1979, vol. 57, # 18, p. 2426 - 2433摘要:DOI:
-
作为产物:描述:参考文献:名称:用于将卤化乙烯转化为α-卤代酮的温和,方便且廉价的方法。摘要:用市售的次氯酸钠水溶液在乙酸/丙酮的2:5混合物中于0摄氏度下处理氯乙烯约1小时,可干净地得到相应的α-氯酮。类似地,如果将溴化乙烯在0摄氏度于2:5乙酸/丙酮作为溶剂中暴露于次溴酸钠(由溴和氢氧化钠新制)中,则会制得α-溴代酮。该方法已经应用于许多氯乙烯和溴乙烯,并且转化通常以高收率进行。温和的反应条件与各种官能团兼容,包括酰胺,酯和亚胺。DOI:10.1021/jo020739m
-
作为试剂:描述:参考文献:名称:Tschesche; Schaefer, Chemische Berichte, 1955, vol. 88, p. 81,87摘要:DOI:
文献信息
-
BENZOTHIOPHENE INHIBITORS OF RHO KINASE申请人:Kahraman Mehmet公开号:US20080021026A1公开(公告)日:2008-01-24The present invention relates to compounds and methods which may be useful as inhibitors of Rho kinase for the treatment or prevention of disease.本发明涉及化合物和方法,这些化合物和方法可能作为Rho激酶的抑制剂在治疗或预防疾病方面有用。
-
[EN] CRBN LIGANDS AND USES THEREOF<br/>[FR] LIGANDS CRBN ET LEURS UTILISATIONS申请人:KYMERA THERAPEUTICS INC公开号:WO2019140387A1公开(公告)日:2019-07-18The present invention provides compounds, compositions thereof, and methods of using the same for the inhibition of CRBN, and the treatment of CRBN-mediated disorders.本发明提供了化合物、其组合物以及使用这些化合物抑制CRBN并治疗CRBN介导的疾病的方法。
-
Guanidinium Ylide Mediated Aziridination from Arylaldehydes: Scope and Limitations in the Formation of Unactivated 3-Arylaziridine-2-carboxylates作者:Tsutomu Ishikawa、Yukiko Oda、Kihito Hada、Marie Miyata、Chisato Takahata、Yukiko Hayashi、Masato Takahashi、Naoki Yajima、Makiko FujinamiDOI:10.1055/s-0033-1341233日期:——Abstract The scope and limitations of guanidinium ylide mediated aziridinations from arylaldehydes yielding unactivated 3-arylaziridine-2-carboxylates, applicable to asymmetric synthesis, are discussed. The scope and limitations of guanidinium ylide mediated aziridinations from arylaldehydes yielding unactivated 3-arylaziridine-2-carboxylates, applicable to asymmetric synthesis, are discussed.
-
[EN] COMPOUNDS<br/>[FR] COMPOSÉS申请人:GLAXOSMITHKLINE IP DEV LTD公开号:WO2018137593A1公开(公告)日:2018-08-02Provided are novel compounds that inhibit LRRK2 kinase activity, processes for their preparation, compositions containing them and their use in the treatment of or prevention of diseases associated with or characterized by LRRK2 kinase activity, for example Parkinson's disease, Alzheimer's disease and amyotrophic lateral sclerosis (ALS).提供了抑制LRRK2激酶活性的新化合物,以及它们的制备方法、含有它们的组合物以及它们在治疗或预防与LRRK2激酶活性相关或以其为特征的疾病中的用途,例如帕金森病、阿尔茨海默病和肌萎缩侧索硬化症(ALS)。
-
New quinoline- and isoquinoline-based multicomponent methods for the synthesis of 1,1(3,3)-dicyanotetrahydrobenzoindolizines作者:I. A. Sanin、A. A. Zubarev、A. Yu. Rudenko、L. A. Rodinovskaya、E. A. Batuev、A. M. ShestopalovDOI:10.1007/s11172-018-2073-z日期:2018.2multicomponent methods for the synthesis of benzannulated dihydroindolizines based on quinoline or isoquinoline, malononitrile, aromatic aldehydes and α-halomethylcarbonyl compounds were developed. Several alternative protocols of using the reactants were studied, starting with separate generation of two most probable intermediates and ending with the four-component condensation of all reactants. The scope
表征谱图
-
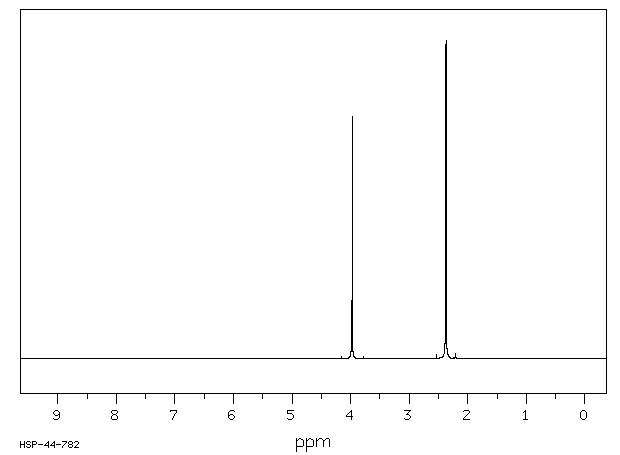
氢谱1HNMR
-
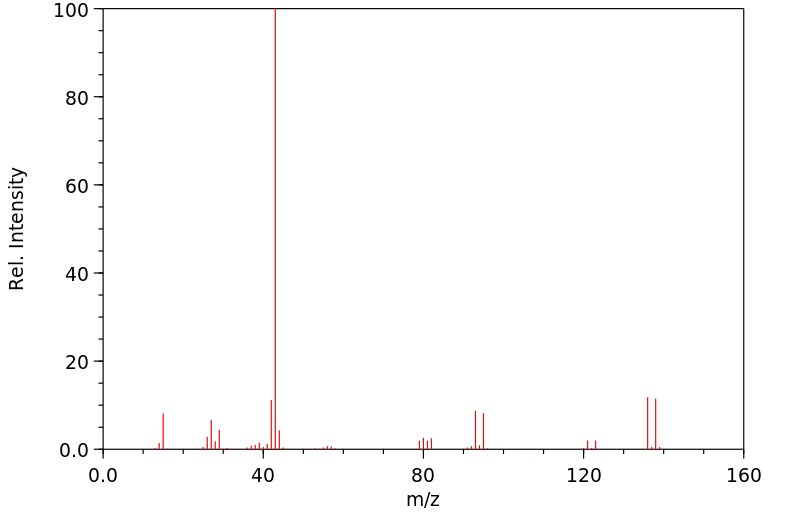
质谱MS
-
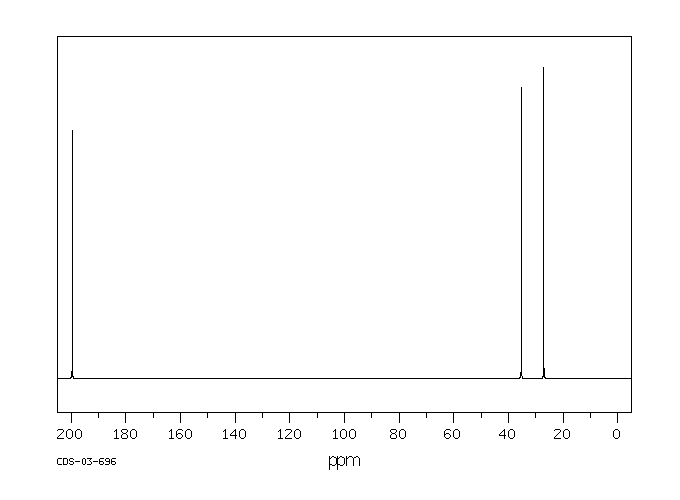
碳谱13CNMR
-
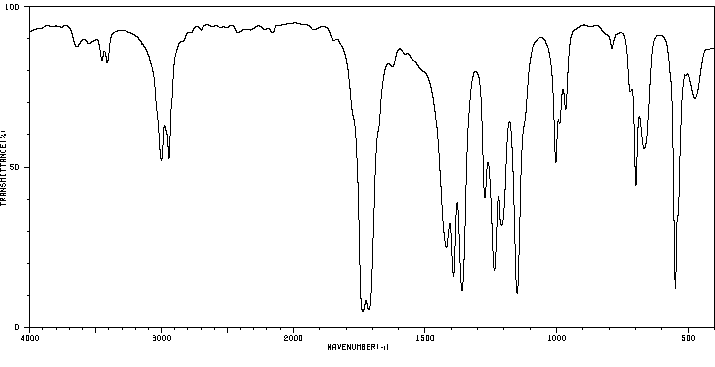
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷