2-氨基-4-(3,4-二氯苯基)噻唑 | 39893-80-6
中文名称
2-氨基-4-(3,4-二氯苯基)噻唑
中文别名
噻唑-2-胺,4-(3,4-二氯苯基)-;4-(3,4-二氯苯基)-1,3-噻唑-2-胺;4-(3,4-二氯苯基)噻唑-2-胺;4-(3,4-二氯苯基)-2-硫佐拉敏;[4-(3,4-二氯苯基)噻唑-2-基]胺
英文名称
4-(3,4-dichloro-phenyl)-thiazol-2-ylamine
英文别名
4-(3,4-dichlorophenyl)thiazol-2-amine;2-amino-4-(3,4-dichlorophenyl)thiazole;4-(3,4-Dichlorophenyl)-1,3-thiazol-2-amine
CAS
39893-80-6
化学式
C9H6Cl2N2S
mdl
MFCD00466323
分子量
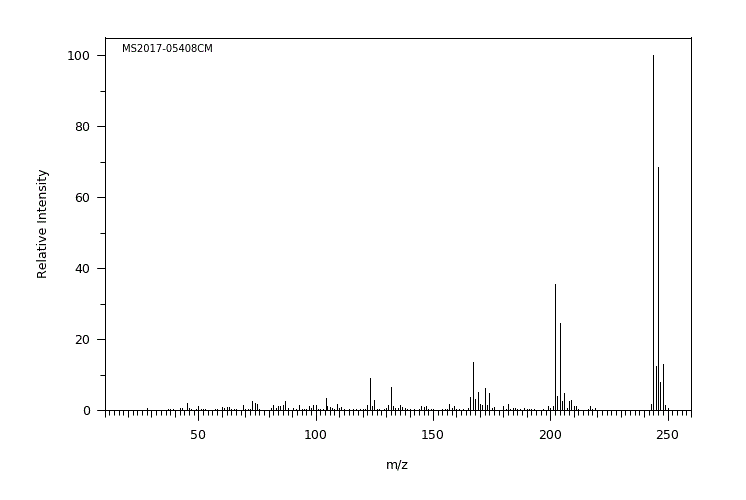
245.132
InChiKey
UWAXMUSNRIUHRQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:161.0 to 165.0 °C
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:67.2
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
海关编码:2934100090
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:2-8°C
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 4-(3,4-Dichloro-phenyl)-thiazol-2-ylamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(3,4-Dichloro-phenyl)-thiazol-2-ylamine
CAS number: 39893-80-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H6Cl2N2S
Molecular weight: 245.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 4-(3,4-Dichloro-phenyl)-thiazol-2-ylamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 4-(3,4-Dichloro-phenyl)-thiazol-2-ylamine
CAS number: 39893-80-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H6Cl2N2S
Molecular weight: 245.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride, sulfur oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]acetamide —— C11H8Cl2N2OS 287.169 —— N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]propanamide —— C12H10Cl2N2OS 301.2 —— 2-cyano-N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]acetamide —— C12H7Cl2N3OS 312.179 —— N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]cyclopropanecarboxamide —— C13H10Cl2N2OS 313.207 —— 4-((4-(3,4-dichlorophenyl)thiazol-2-yl)amino)-4-oxobutanoic acid 560090-53-1 C13H10Cl2N2O3S 345.206 —— N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]-3-pyridinecarboxamide —— C15H9Cl2N3OS 350.228 —— 6-chloro-N2-[4-(3,4-dichlorophenyl)thiazol-2-yl]-N4,N4-diethyl-1,3,5-triazine-2,4-diamine 1366099-15-1 C16H15Cl3N6S 429.76 —— N-[4-(3,4-dichlorophenyl)-1,3-thiazol-2-yl]-2,6-difluorobenzamide —— C16H8Cl2F2N2OS 385.221 —— 5-chloro-2-hydroxy-N-[4-(3,4-dichlorophenyl)thiazol-2-yl]benzamide 634186-92-8 C16H9Cl3N2O2S 399.685
反应信息
-
作为反应物:描述:2-氨基-4-(3,4-二氯苯基)噻唑 在 碘 、 碘酸 作用下, 以 水 为溶剂, 反应 0.18h, 生成 4-(3,4-dichloro-phenyl)-5-iodo-thiazol-2-ylamine参考文献:名称:一种快速、温和、高效的 2-氨基噻唑 C-5 碘化/硫氰化方法摘要:图形摘要 摘要 开发了一种高效、绿色、快速的多任务协议,用于使用碘酸和 PEG-400 水溶液选择性地 C-5 取代 2-氨基噻唑。该方法发现适用于 C-5 取代,即分别使用碘和硫氰酸铵对 2-氨基噻唑进行碘化和硫氰化。碘酸被发现是一种良好的氧化剂,PEG-400 水溶液作为绿色反应溶剂。DOI:10.1080/10426507.2016.1149851
-
作为产物:描述:参考文献:名称:柠檬酸一锅中通过C–Br,C–S和C–N键的形成从酮中合成2,4-二取代的噻唑:一种绿色方法摘要:已开发出一种改进的,更环保的方案,可通过一步一步从现成的酮类N-溴代琥珀酰亚胺(NBS),C-Br,C-S和C-N键的形成中合成2,4-二取代的噻唑,在回流条件下,柠檬酸在乙醇和水(3:1)的混合物中由柠檬酸催化硫脲。该方法的优点是无需分离催泪性α-溴代酮,易于操作,反应曲线更干净,底物范围宽,无需色谱纯化和适合大规模合成。DOI:10.1002/jccs.201700200
文献信息
-
[EN] GLYCOLATE OXIDASE INHIBITORS FOR THE TREATMENT OF DISEASE<br/>[FR] INHIBITEURS DE GLYCOLATE OXYDASE POUR LE TRAITEMENT D'UNE MALADIE申请人:BIOMARIN PHARM INC公开号:WO2020257487A1公开(公告)日:2020-12-24Described herein are compounds, methods of making such compounds, pharmaceutical compositions and medicaments containing such compounds, and methods of using such compounds to treat or prevent diseases or disorders associated with a defect in glyoxylate metabolism, for example a disease or disorder associated with the enzyme glycolate oxidase (GO) or alterations in oxalate metabolism. Such diseases or disorders include, for example, disorders of glyoxylate metabolism, including primary hyperoxaluria, that are associated with production of excessive amounts of oxalate.
-
N-(5-MEMBERED AROMATIC RING)-AMIDO ANTI-VIRAL COMPOUNDS申请人:Schmitz Ulrich Franz公开号:US20070265265A1公开(公告)日:2007-11-15Disclosed are compounds having Formula (I) and the compositions and methods thereof for treating or preventing a viral infection mediated at least in part by a virus in the Flaviviridae family of viruses, wherein A, R 2 , m, R, V, W, T, Z, R 1 , Y, and p are disclosed herein.揭示了具有Formula (I)的化合物,以及用于治疗或预防由Flaviviridae病毒家族中的病毒至少部分介导的病毒感染的组合物和方法,其中A、R2、m、R、V、W、T、Z、R1、Y和p在此处被揭示。
-
Synthesis, activity, and docking study of phenylthiazole acids as potential agonists of PPAR&gamma;作者:Liang Ma、Taijin Wang、Min Shi、Haoyu YeDOI:10.2147/dddt.s106406日期:——glucose and lipid homeostasis, and PPARγ ligands possess therapeutic potential in these as well as other areas. In this study, a series of phenylthiazole acids have been synthesized and evaluated for agonistic activity by a convenient fluorescence polarization-based PPARγ ligand screening assay. Compound 4t, as a potential PPARγ agonist with half maximal effective concentration (EC50) 0.75±0.20 μM, exhibited
-
Synthesis, characterization, biological activity, and 3D-QSAR studies on some novel class of pyrrole derivatives as antitubercular agents作者:Shrinivas D. Joshi、Uttam A. More、Sheshagiri R. Dixit、Haresh H. Korat、Tejraj M. Aminabhavi、Aravind M. BadigerDOI:10.1007/s00044-013-0709-y日期:2014.3designed, synthesized, and their structures have been elucidated along with the evaluation of antitubercular activity against Mycobacterium tuberculosis H37Rv using the microplate alamar blue assay method and antibacterial activity against Staphylococcus aureus, Bacillus subtilis, Klebsiella pneumoniae, and Escherichia coli by broth micro-dilution assay method. Structural activity relationships and 3D-QSAR
-
Solid state thiazole-based fluorophores: Promising materials for white organic light emitting devices作者:Kumar Godugu、Sultana Shaik、Mohammad Khaja Mohinuddin Pinjari、Trivikram Reddy Gundala、Dwaraka Viswanath Chellappa Subramanyam、Subramanyam Sarma Loka、Haranath Divi、Vemula Venkatramu、Chinna Gangi Reddy NallagonduDOI:10.1016/j.dyepig.2020.109077日期:2021.3solid state white light emissive thiazole-based donor-acceptor (D-A) type fluorophores, 2-(3-pyridyl)/2-aminothiazoles from ω-bromomethylketones and pyridine-3-carbothioamide/thiourea in the presence of silica-supported HClO4 as a reusable solid Brønsted acid catalyst at RT. The photophysical and electrochemical properties of these compounds have been derived. Most of the studied D-A type solid thiazole-based已开发出一种简便且更有效的无溶剂机械化学合成路线,用于合成一系列固态白光发射的噻唑基供体-受体(DA)型荧光团,2-(3-吡啶基)/ 2-氨基噻唑在室温下,作为可重复使用的固体布朗斯台德酸催化剂,在二氧化硅负载的HClO 4存在下,ω-溴甲基酮和吡啶-3-碳硫酰胺/硫脲。已经推导了这些化合物的光物理和电化学性质。大多数研究过的DA型基于噻唑的固体荧光团都发出白光,并且可以通过在第4个引入各种取代基从温暖的(理想的)冷的白光进行调谐。2-(3-吡啶基)/ 2-氨基噻唑的位置。此外,发现标题化合物的HOMO和LUMO能级分别在-5.52eV至-5.72eV和-1.84eV至-2.45eV的范围内。这些水平的噻唑基荧光团的寿命已通过发光衰减曲线确定,发现范围为7.7–11μs。合成的噻唑基荧光团的光物理和电化学性质表明,该化合物可能是用于白色有机发光器件的有前途的材料。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
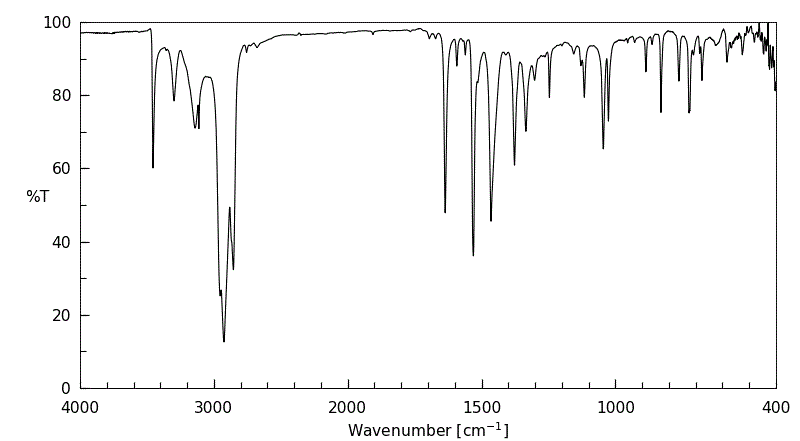
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫