3,5-二硝基苯甲酸甲酯 | 2702-58-1
中文名称
3,5-二硝基苯甲酸甲酯
中文别名
S(-)-α-甲基苄胺;乙酰环丙孕酮醋酸酯
英文名称
methyl 3,5-dinitrobenzoate
英文别名
3,5-dinitrobenzoic acid methyl ester
CAS
2702-58-1
化学式
C8H6N2O6
mdl
MFCD00017016
分子量
226.145
InChiKey
POGCCFLNFPIIGW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:107-109 °C (lit.)
-
沸点:367.74°C (rough estimate)
-
密度:1.6136 (rough estimate)
-
保留指数:1690;1694;1701;1714;1724;1745
-
稳定性/保质期:
遵照规定使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:16
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:118
-
氢给体数:0
-
氢受体数:6
安全信息
-
危险品标志:Xn
-
安全说明:S24/25
-
危险类别码:R22
-
WGK Germany:3
-
海关编码:2916399090
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:存于阴凉干燥处。
SDS
| Name: | Methyl 3 5-dinitrobenzoate 99% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 2702-58-1 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 2702-58-1 | Methyl 3,5-dinitrobenzoate | 99 | 220-289-9 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Vacuum or sweep up material and place into a suitable disposal container. Clean up spills immediately, observing precautions in the Protective Equipment section. Avoid generating dusty conditions.
Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Use with adequate ventilation.
Minimize dust generation and accumulation. Avoid breathing dust, vapor, mist, or gas. Avoid contact with eyes, skin, and clothing.
Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 2702-58-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 107 - 109 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H6N2O6
Molecular Weight: 226.15
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Nitrogen oxides, carbon monoxide, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 2702-58-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
Methyl 3,5-dinitrobenzoate - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 2702-58-1: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 2702-58-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 2702-58-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途:用于有机合成中间体。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 3,5-二硝基苯甲酸 3,5-Dinitrobenzoic acid 99-34-3 C7H4N2O6 212.119 —— 3,5-dinitrobenzoic anhydride 40993-10-0 C14H6N4O11 406.222 3-硝基苯甲酸甲酯 methyl 3-nitrobenzoate 618-95-1 C8H7NO4 181.148 3,5-二硝基苯甲醛 3,5-dinitrobenzaldehyde 14193-18-1 C7H4N2O5 196.119 3,5-二硝基苯甲酰氯 3,5-dinitrobenoyl chloride 99-33-2 C7H3ClN2O5 230.564 —— 3,5-dinitrobenzoyl azide 42444-51-9 C7H3N5O5 237.131 3,5-二硝基苯甲腈 3,5-dinitrobenzonitrile 4110-35-4 C7H3N3O4 193.119 —— 1,3-dinitro-5-trichloromethylbenzene 90325-19-2 C7H3Cl3N2O4 285.471 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-氨基-5-硝基苯甲酸甲酯 3-amino-5-nitro-benzoic acid methyl ester 23218-93-1 C8H8N2O4 196.163 3,5-二硝基苯甲酸乙酯 ethyl 3,5-dinitrobenzoate 618-71-3 C9H8N2O6 240.172 3,5-二硝基苯甲酸 3,5-Dinitrobenzoic acid 99-34-3 C7H4N2O6 212.119 3-乙酰氨基-5-硝基苯甲酸甲酯 methyl 3-acetamido-5-nitrobenzoate 14622-17-4 C10H10N2O5 238.2 3-碘-5-硝基苯甲酸甲酯 methyl 3-iodo-5-nitrobenzoate 50765-19-0 C8H6INO4 307.044 3,5-二硝基苯甲酰胺 3,5-dinitrobenzamide 121-81-3 C7H5N3O5 211.134 —— 3-methylsulfanyl-5-nitrobenzoic acid methyl ester 1260491-64-2 C9H9NO4S 227.241 3,5-二氨基苯甲酸甲酯 methyl 3,5-diaminobenzoate 1949-55-9 C8H10N2O2 166.18 3,5-二硝基苯甲酰肼 3,5-dinitrobenzohydrazide 2900-63-2 C7H6N4O5 226.148 3-羟基-5-硝基苯甲酸 3-hydroxy-5-nitrobenzoic acid 78238-14-9 C7H5NO5 183.12 3-甲氧基5-硝基苯甲酸乙酯 3-methoxy-5-nitrobenzoic acid methyl ester 78238-13-8 C9H9NO5 211.174 —— N-(2-hydroxyethyl)-3,5-dinitrobenzamide 79883-94-6 C9H9N3O6 255.187 3-氨基-5-硝基苯甲酰肼 3-amino-5-nitrobenzohydrazide 205652-98-8 C7H8N4O3 196.166 3-甲氧基-5-硝基苯甲酸 3-methoxy-5-nitrobenzoic acid 78238-12-7 C8H7NO5 197.147 —— methyl 3-(4-fluorophenoxy)-5-nitrobenzoate 1428901-25-0 C14H10FNO5 291.236 —— 1-(1-Adamantyl)-3-methoxycarbonyl-5-nitrobenzol 71466-55-2 C18H21NO4 315.369 3,5-二氨基苯甲酸 3.5-diaminobenzoic acid 535-87-5 C7H8N2O2 152.153 - 1
- 2
反应信息
-
作为反应物:参考文献:名称:设计,合成和生物学评价米-amidophenol衍生物作为一类新的抗结核剂的†摘要:一系列的米-amidophenol衍生物(6A-6L,图7a-7Q,图9a,图9b,图12A-12C,14和15),设计并合成。他们的抗结核活性进行了评价在体外对结核分枝杆菌菌株和杆菌H37Ra和H37Rv的临床分离耐多药的结核分枝杆菌菌株。十种化合物在2.5μgmL -1以下对结核分枝杆菌H37Ra表现出最低抑菌浓度(MICs),而6g是活性最高的化合物(MIC = 0.625μgmL -1)。化合物6g和7a还显示出对结核分枝杆菌H37Rv (MIC = 0.39μgmL -1)和几种临床分离的耐多药结核分枝杆菌菌株(MIC = 0.39-3.125μgmL -1)的有效抑制活性。该化合物对正常的革兰氏阳性和革兰氏阴性细菌没有抑制活性。他们表现出对HepG2和RAW264.7细胞系的低细胞毒性。结果表明米-amidophenol作为新的抗结核药物开发有吸引力的支架。DOI:10.1039/c8md00212f
-
作为产物:描述:参考文献:名称:Aizpurua, Jesus Mari; Palomo, Claudio, Synthesis, 1982, # 8, p. 684 - 686摘要:DOI:
文献信息
-
Design of photoaffinity labeling probes derived from 3,4,5-trimethylfuran-2(5 H )-one for mode of action elucidation作者:Martin Pošta、Vilmos Soós、Petr BeierDOI:10.1016/j.tet.2016.03.096日期:2016.7Herein we report the synthesis of new probes for photoaffinity labeling with the aim of receptor identification and mode of action elucidation of 3,4,5-trimethylfuran-2(5H)-one (TMB), recently identified in the smoke of burning vegetation as an efficient seed germination inhibitor. These photoaffinity probes consist of an ethynyl group that acts as a tag for introduction of an optional detectable marker
-
Zinc phthalocyanine with PEG-400 as a recyclable catalytic system for selective reduction of aromatic nitro compounds作者:Upendra Sharma、Neeraj Kumar、Praveen Kumar Verma、Vishal Kumar、Bikram SinghDOI:10.1039/c2gc35452g日期:——for the first time. The present catalytic system was successfully employed for the reduction of carbonyl and ester compounds to corresponding alcohols and reductive amination of benzaldehydes with primary amines to form corresponding secondary amines. Remarkable advantages of the present catalytic method include low loading of metal, avoidance of toxic ligands and high isolated yields. The catalyst was
-
Synthesis and Evaluation of Some Substituted Heterocyclic Fluconazole Analogues as Antifungal Agents作者:Shudong Wang、Lei Zhang、Yongsheng Jin、Jin Hao Tang、Hua Su、Shichong Yu、Haixiang RenDOI:10.14233/ajchem.2014.15956日期:——A new series of fluconazole analogues of 1-(1H-1,2,4-triazol-1-yl)-2-(2,4-difluoro-phenyl)-3-4-(substituted-heterocyclic ring-1H-1,2,3-triazol-1-yl)-2-propanols (1-10) were designed, synthesized and evaluated as antifungal agents. Preliminary antifungal tests showed that most of the title compounds exhibited moderate activity with broad spectrum against eight human pathogenic fungi in vitro, compounds 1 and 6 had the best antifungal activity against Candida albicans with the value of MIC80 = 0.5 μg/mL respectively.设计、合成了1-(1H-1,2,4-三唑-1-基)-2-(2,4-二氟苯基)-3-4-(取代杂环-1H-1,2,3-三唑-1-基)-2-丙醇(1-10)系列氟康唑类似物,并评价了其抗真菌活性。初步抗真菌测试显示,大多数标题化合物对8种人体致病真菌具有中等活性且谱广,其中化合物1和6对白色念珠菌显示出最佳的抗真菌活性,MIC80值分别达到0.5 μg/mL。
-
[EN] (3-HYDROXY-4-AMINO-BUTAN-2-YL) -3- (2-THIAZOL-2-YL-PYRROLIDINE-1-CARBONYL) BENZAMIDE DERIVATIVES AND RELATED COMPOUNDS AS BETA-SECRETASE INHIBITORS FOR TREATING<br/>[FR] DÉRIVÉS DE (3-HYDROXY-4-AMINO-BUTAN-2-YL) -3- (2-THIAZOL-2-YL-PYRROLIDINE-1-CARBONYL) BENZAMIDE ET COMPOSÉS ASSOCIÉS UTILISÉS EN TANT QU'INHIBITEURS DE LA BÊTA-SÉCRÉTASE POUR LE TRAITEMENT申请人:COMENTIS INC公开号:WO2009042694A1公开(公告)日:2009-04-02The present invention provides novel beta-secretase inhibitors and methods for their use, including methods of treating of Alzheimer's disease. (Formula)本发明提供了新颖的β-分泌酶抑制剂及其使用方法,包括用于治疗阿尔茨海默病的方法。
-
Relative reactivity of methyl iodide to ethyl iodide in nucleophilic substitution reactions in acetonitrile and partial desolvation accompanying activation作者:Yasuhiko Kondo、Miyuki Urade、Yukari Yamanishi、Xinyu ChenDOI:10.1039/b203032m日期:——have been deduced on the basis of these classifications. A major factor determining the relative reactivity of methyl iodide to ethyl iodide in the substitution reaction of an anionic nucleophile having a single reaction site in acetonitrile (kMeI/kEtI) is suggested to be partial desolvation around the nucleophilic center on going from reactant to transition-state.
表征谱图
-
氢谱1HNMR
-
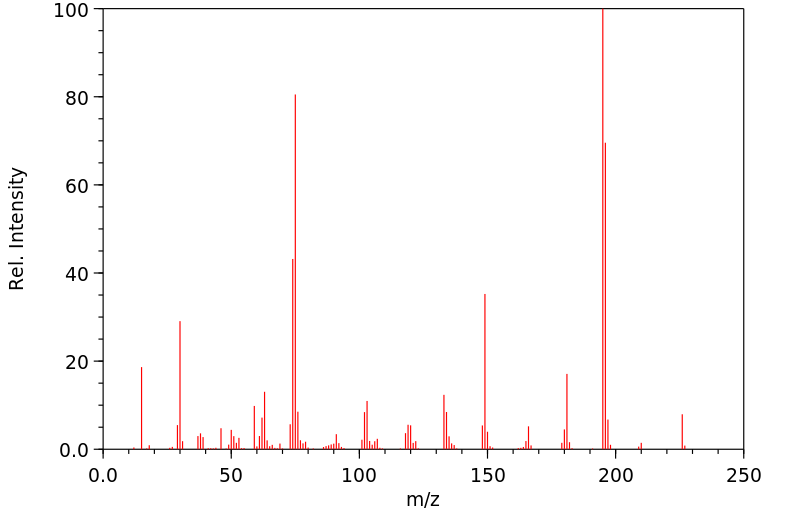
质谱MS
-
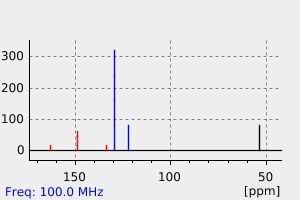
碳谱13CNMR
-
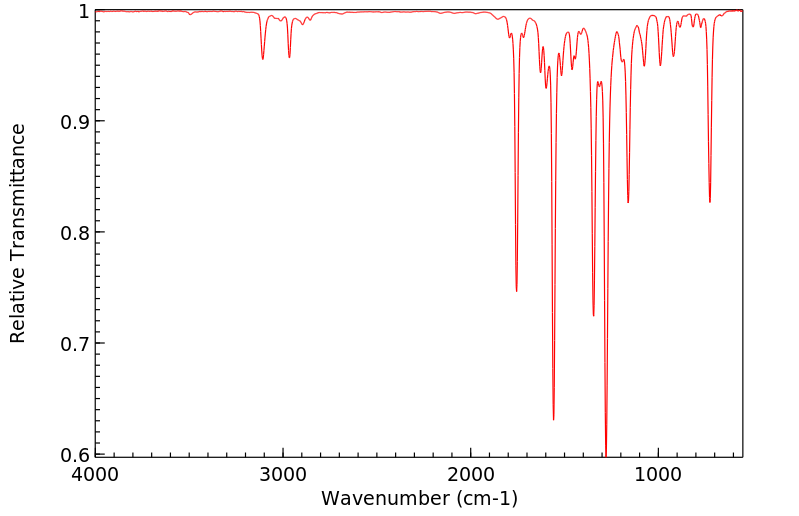
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫