3-氟苯甲酸甲酯 | 455-68-5
中文名称
3-氟苯甲酸甲酯
中文别名
间氟苯甲酸甲酯
英文名称
methyl 3-fluorobenzoate
英文别名
3-fluorobenzoic acid methyl ester
CAS
455-68-5
化学式
C8H7FO2
mdl
MFCD03094302
分子量
154.141
InChiKey
YXZNVLYXBIIIOB-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-10 °C
-
沸点:194-195 °C
-
密度:1.171±0.06 g/cm3(Predicted)
-
溶解度:可溶于丙酮、DMSO、乙酸乙酯
-
保留指数:1074
计算性质
-
辛醇/水分配系数(LogP):2.3
-
重原子数:11
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.125
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:3
安全信息
-
危险品标志:T
-
海关编码:2916399090
-
储存条件:| 室温 |
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: Methyl 3-fluorobenzoate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 3-fluorobenzoate
CAS number: 455-68-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7FO2
Molecular weight: 154.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: Methyl 3-fluorobenzoate
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Methyl 3-fluorobenzoate
CAS number: 455-68-5
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H7FO2
Molecular weight: 154.1
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen fluoride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 间氟苯甲酸 3-fluorobenzoic acid 455-38-9 C7H5FO2 140.114 4-氯-3-氟苯甲酸甲酯 methyl 4-chloro-3-fluorobenzoate 206362-87-0 C8H6ClFO2 188.586 2-碘-5-氟苯甲酸甲酯 methyl 5-fluoro-2-iodobenzoate 1202897-48-0 C8H6FIO2 280.037 苯甲酸甲酯 benzoic acid methyl ester 93-58-3 C8H8O2 136.15 3-氟苄醇 3-fluoro-benzenemethanol 456-47-3 C7H7FO 126.13 3-氟苯甲醛 3-Fluorobenzaldehyde 456-48-4 C7H5FO 124.115 邻氟苯甲酸 2-Fluorobenzoic acid 445-29-4 C7H5FO2 140.114 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 3-氟苯甲酸乙酯 ethyl 3-fluorobenzoate 451-02-5 C9H9FO2 168.168 间氟苯甲酸 3-fluorobenzoic acid 455-38-9 C7H5FO2 140.114 —— [18F]methyl 3-fluoro-4-fluorobenzoate —— C8H6F2O2 171.133 3-氟-4-羟基苯甲酸甲酯 methyl 3-fluoro-4-hydroxybenzoate 403-01-0 C8H7FO3 170.14 3-氟-5-羟基苯甲酸甲酯 methyl 3-fluoro-5-hydroxybenzoate 1072004-32-0 C8H7FO3 170.14 5-氟-2-羟基苯甲酸甲酯 methyl 5-fluoro-2-hydroxybenzoate 391-92-4 C8H7FO3 170.14 3-氟-2-羟基-苯甲酸甲酯 methyl 3-fluoro-2-hydroxybenzoate 70163-98-3 C8H7FO3 170.14 苯甲酸乙酯 benzoic acid ethyl ester 93-89-0 C9H10O2 150.177 3-氟苄醇 3-fluoro-benzenemethanol 456-47-3 C7H7FO 126.13 —— 2-fluoro-6-(methoxycarbonyl)benzoic acid 1256593-39-1 C9H7FO4 198.151
反应信息
-
作为反应物:参考文献:名称:通过铜催化的原脱碳甲氧基化还原还原的芳香和杂芳族酯功能。摘要:使用廉价,无毒且空气稳定的Cu(OAc)2催化剂成功实现了芳香族和杂芳香族甲基酯功能的空前催化还原裂解。该反应快速,具有良好的官能团耐受性,不需要惰性气氛或无水溶剂,并且可以按比例放大至1 g。此外,在这些条件下,羧酸和叔丁酯也能平滑地反应。DOI:10.1021/acs.orglett.8b00930
-
作为产物:参考文献:名称:作为 FtsZ 抑制剂的靛红衍生物的设计、合成和抗菌活性摘要:摘要 设计了七种靛红衍生物,并通过单晶X射线衍射、 1 H NMR、MS和元素分析对其化学结构进行了表征。结构稳定,然后是分子内和分子间 H 键,使这些分子成为分子识别中与单个分子内的自互补供体和受体单元的完美例子。评价这些化合物的抗微生物活性。已执行对接模拟以将化合物定位到 FtsZ 活性位点,以确定它们可能的结合模型。所有化合物均表现出较好的抗菌活性。有趣的是,化合物5c和5d对金黄色葡萄球菌表现出更好的抗菌活性,IC 50 值分别为0.03和0.05 μmol/mL。DOI:10.1016/j.molstruc.2016.03.036
文献信息
-
Esterification of Aryl/Alkyl Acids Catalysed by N-bromosuccinimide under Mild Reaction Conditions作者:Klara Čebular、Bojan Božić、Stojan StavberDOI:10.3390/molecules23092235日期:——(NBS) has been promoted as the most efficient and selective catalyst among the NXSs in the reaction of direct esterification of aryl and alkyl carboxylic acids. Comprehensive esterification of substituted benzoic acids, mono-, di- and tri-carboxy alkyl derivatives has been performed under neat reaction conditions. The method is metal-free, air- and moisture-tolerant, allowing for a simple synthetic and
-
A simple reduction of methyl aromatic esters to alcohols using sodium borohydride–methanol system作者:Núbia Boechat、Jorge Carlos Santos da Costa、Jorge de Souza Mendonça、Pedro Santos Mello de Oliveira、Marcus Vinı́cius Nora De SouzaDOI:10.1016/j.tetlet.2004.06.034日期:2004.7Several aromatic esters were reduced to the corresponding alcohol by using sodium borohydride–methanol system. The reduction was completed within 2.0–4.0 h after refluxing in THF. The alcohol products were isolated after aqueous workup in good yields (88–97%).
-
Cooperative interplay between a flexible PNN-Ru(<scp>ii</scp>) complex and a NaBH<sub>4</sub> additive in the efficient catalytic hydrogenation of esters作者:Zheng Wang、Xiangyang Chen、Bo Liu、Qing-bin Liu、Gregory A. Solan、Xinzheng Yang、Wen-Hua SunDOI:10.1039/c6cy02413k日期:——A catalyst loading of between 0.001–0.05 mol% of the PNN-bearing ruthenium(II) complex [fac-PNN]RuH(PPh3)(CO) (PNN = 8-(2-diphenylphosphinoethyl)amidotrihydroquinoline), in combination with 5 mol% NaBH4, efficiently catalyzes the hydrogenation of esters to their corresponding alcohols under mild pressures of hydrogen. Both aromatic and aliphatic esters can be converted with high values of TON or TOF催化剂负载量为含PNN的钌(II)配合物[ fac -PNN] RuH(PPh 3)(CO)(PNN = 8-(2-二苯基膦基乙基)氨基三氢喹啉)的0.001-0.05 mol%摩尔%的NaBH 4可以在温和的氢气压力下有效催化酯加氢成相应的醇。芳族和脂族酯都可以以高的TON或TOF值转化。使用DFT计算和标记实验进行的机理研究突显了NaBH 4在催化中的协同作用,而催化活性物质已被确定为反式-二氢化物[ mer -PN HN] RuH 2(CO)(PN H N = 8-(2-二苯基膦乙基)氨基三氢喹啉)。PN H N-钌物种的立体结构极大地影响了催化剂的活性,实际上,顺式-二氢异构体[ fac -PN H N] RuH 2(CO)不能催化酯的氢化,直到配体重组为止发生以得到反式异构体。
-
Acylation of oxindoles using methyl/phenyl esters <i>via</i> the mixed Claisen condensation – an access to 3-alkylideneoxindoles作者:Ramdas Sreedharan、Purushothaman Rajeshwaran、Pradeep Kumar Reddy Panyam、Saurabh Yadav、C. M. Nagaraja、Thirumanavelan GandhiDOI:10.1039/d0ob00789g日期:——Predominantly, aggressive acid chlorides and stoichiometric coupling reagents are employed in the acylating process for synthesizing carbonyl tethered heterocycles. Herein, we report simple acyl sources, viz. methyl and phenyl esters, which acylate oxindoles via the mixed Claisen condensation. This straightforward protocol is mediated by LiHMDS and KOtBu and successfully applied to a wide range of
-
Discovery of Multicomponent Heterogeneous Catalysts via Admixture Screening: PdBiTe Catalysts for Aerobic Oxidative Esterification of Primary Alcohols作者:David S. Mannel、Maaz S. Ahmed、Thatcher W. Root、Shannon S. StahlDOI:10.1021/jacs.6b12722日期:2017.2.1effective for aerobic oxidative methyl esterification of primary alcohols. The identification of possible catalysts for this reaction was initiated by the screening of simple binary and ternary admixtures of Pd/charcoal in combination with one or two metal and/or metalloid components as the catalyst. This approach permitted rapid evaluation of over 400 admixture combinations for the oxidative methyl esterification在本研究中,我们展示了“混合物筛选”在发现新的多组分多相 Pd 催化剂组合物方面的效用,这些组合物对伯醇的有氧氧化甲酯化非常有效。该反应可能的催化剂的鉴定是通过筛选简单的二元和三元 Pd/木炭混合物与一种或两种金属和/或准金属组分作为催化剂而开始的。这种方法允许快速评估 400 多种混合物组合,用于在 60 °C 的甲醇中氧化甲基酯化 1-辛醇。这些反应的产物产率变化很大,范围从 2% 到 88%。观察到基于 Bi、Te 和 Pb 的添加剂的产量最高,尤其是那些同时含有 Bi 和 Te 的添加剂。通过湿浸法制备特定的 PdBiTe 催化剂配方,然后应用响应面方法来确定最佳的 Pd-Bi-Te 催化剂化学计量,从而对结果进行验证。这种方法揭示了两种非常有效的催化剂组合物:PdBi0.47Te0.09/C (PBT-1) 和 PdBi0.35Te0.23/C (PBT-2)。前一种催化剂用于与
表征谱图
-
氢谱1HNMR
-
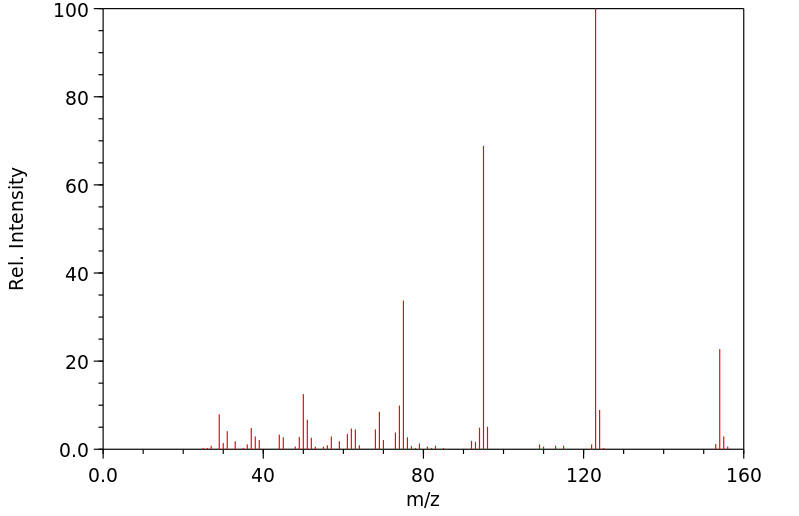
质谱MS
-
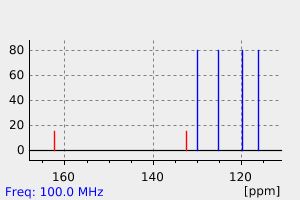
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫