N-[2-(2-Benzyloxy-3,4-dimethoxy-phenyl)-ethyl]-2-(4-benzyloxy-3-methoxy-phenyl)-acetamide | 35178-88-2
中文名称
——
中文别名
——
英文名称
N-[2-(2-Benzyloxy-3,4-dimethoxy-phenyl)-ethyl]-2-(4-benzyloxy-3-methoxy-phenyl)-acetamide
英文别名
N-[2-(3,4-dimethoxy-2-phenylmethoxyphenyl)ethyl]-2-(3-methoxy-4-phenylmethoxyphenyl)acetamide
CAS
35178-88-2
化学式
C33H35NO6
mdl
——
分子量
541.644
InChiKey
OUXAJPONXKUULY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:722.6±60.0 °C(Predicted)
-
密度:1.169±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):5.9
-
重原子数:40
-
可旋转键数:14
-
环数:4.0
-
sp3杂化的碳原子比例:0.24
-
拓扑面积:75.2
-
氢给体数:1
-
氢受体数:6
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-(3,4-二甲氧基-2-苯基甲氧基苯基)乙胺 2-Benzyloxy-3,4-dimethoxy-β-phenethylamin 15778-62-8 C17H21NO3 287.359 —— 2-(4-(benzyloxy)-3-methoxyphenyl)acetyl chloride 54313-35-8 C16H15ClO3 290.746
反应信息
-
作为反应物:描述:N-[2-(2-Benzyloxy-3,4-dimethoxy-phenyl)-ethyl]-2-(4-benzyloxy-3-methoxy-phenyl)-acetamide 在 palladium on activated charcoal 氢气 、 sodium hydride 、 碳酸氢钠 、 三氯氧磷 作用下, 以 四氢呋喃 、 乙酸乙酯 、 N,N-二甲基甲酰胺 、 乙腈 为溶剂, 20.0 ℃ 、517.11 kPa 条件下, 反应 21.0h, 生成 lamellarin K参考文献:名称:Total Synthesis of Natural and Unnatural Lamellarins with Saturated and Unsaturated D-Rings摘要:Twenty-eight natural and unnatural lamellarins with either a saturated or an unsaturated D-ring were synthesized according to our developed synthetic route. The key step involved the Michael addition/ring closure (Mi-RC) of the benzyldihydroisoquinoline and alpha-nitrocinnamate derivatives, which provided the 2-carboethoxypyrrole intermediates in moderate to good yields (up to 78% yield). Subsequent hydrogenolysis/lactonization furnished lamellarins with a saturated D-ring in excellent yields (up to 93% yield). DDQ oxidation of the saturated lamellarin acetates led directly to the corresponding unsaturated analogues in 54-95% yield. In addition, only two steps in our developed strategy require column chromatography.DOI:10.1021/jo061810h
-
作为产物:描述:香草醛 在 lithium aluminium tetrahydride 、 sodium carbonate 、 potassium carbonate 、 溶剂黄146 、 乙二胺 作用下, 以 四氢呋喃 、 二氯甲烷 、 水 、 丙酮 为溶剂, 反应 32.0h, 生成 N-[2-(2-Benzyloxy-3,4-dimethoxy-phenyl)-ethyl]-2-(4-benzyloxy-3-methoxy-phenyl)-acetamide参考文献:名称:Total Synthesis of Natural and Unnatural Lamellarins with Saturated and Unsaturated D-Rings摘要:Twenty-eight natural and unnatural lamellarins with either a saturated or an unsaturated D-ring were synthesized according to our developed synthetic route. The key step involved the Michael addition/ring closure (Mi-RC) of the benzyldihydroisoquinoline and alpha-nitrocinnamate derivatives, which provided the 2-carboethoxypyrrole intermediates in moderate to good yields (up to 78% yield). Subsequent hydrogenolysis/lactonization furnished lamellarins with a saturated D-ring in excellent yields (up to 93% yield). DDQ oxidation of the saturated lamellarin acetates led directly to the corresponding unsaturated analogues in 54-95% yield. In addition, only two steps in our developed strategy require column chromatography.DOI:10.1021/jo061810h
文献信息
-
Designing New Analogs for Streamlining the Structure of Cytotoxic Lamellarin Natural Products作者:Kassrin Tangdenpaisal、Rattana Worayuthakarn、Supatra Karnkla、Poonsakdi Ploypradith、Pakamas Intachote、Suchada Sengsai、Busakorn Saimanee、Somsak Ruchirawat、Montakarn ChittchangDOI:10.1002/asia.201403361日期:2015.4Despite the therapeutic potential of marine‐derived lamellarin natural products, their preclinical development has been hampered by their lipophilic nature, causing very poor aqueous solubility. In order to develop more drug‐like analogs, their structure was streamlined in this study from both the cytotoxic activity and lipophilicity standpoints. First, a modified total synthetic route was successfully
表征谱图
-
氢谱1HNMR
-
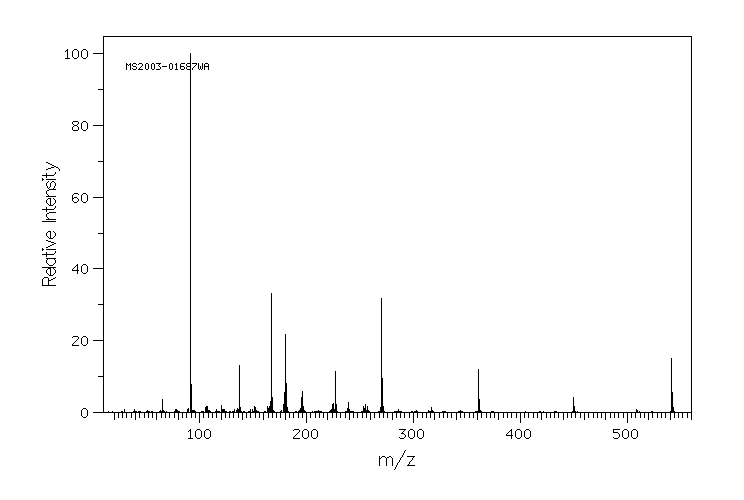
质谱MS
-
碳谱13CNMR
-
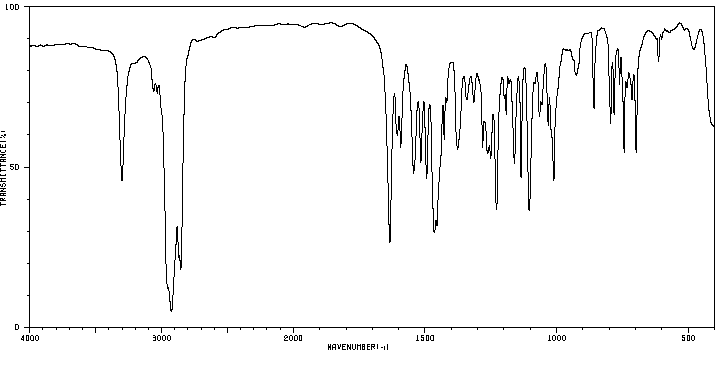
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(N,N-二乙基-4-亚硝基苯胺HYDROCHLOR&)
高石蒜碱
表雪花莲胺碱
表布蕃素
表-加兰它敏N-氧化物
石蒜裂碱
石蒜胺
石蒜碱
盐酸石蒜碱一水合物
盐酸石蒜碱
盐酸加兰他敏
白斑网球花碱
波叶尼润碱
水鬼蕉碱
水鬼蕉碱
水仙环素
氢溴酸加兰他敏
氢氧化六氢11-乙基-6-羟基-3-甲氧基-11-甲基-5,6,9,10,11,12--4aH-[1]苯并呋喃并[3a,3,2-ef][2]苯并吖庚英-11-正离子
条纹碱
文殊兰碱
文殊兰明碱
文拉法辛杂质
小星蒜碱
安贝灵
孤挺花宁碱
多花水仙碱
吗啉,4-[(2R)-3-[4-(1,1-二甲基丙基)苯基]-2-甲基丙基]-2,6-二甲基-,(2R,6S)-
右旋那维啶
右旋加兰他敏
化合物 T30502
加兰它敏-O-甲基-d3
加兰他敏中间体1
加兰他敏N-氧化物
加兰他敏
加兰他敏
二氢石蒜碱
二氢加兰他敏
乙酰基孤挺花宁碱
丁苯海拉明
[13C,2H3]-氢溴酸加兰他敏
SBE13盐酸盐
O-乙酰基加兰它敏
N-甲基-n-(2-[4-羟基苯基]乙基)-2-溴-5-羟基-4-甲氧基苯羧酰胺
N-去甲基加兰它敏氢溴酸盐
N-[2-(3,4-二甲氧基苯基)乙基]-4-苯氧基苯甲酰胺
N-[2-(3,4-二甲氧基苯基)乙基]-3,4-二甲氧基苯甲酰胺
N-[(3,4-二甲氧基苯基)甲基]-4-甲氧基-N-甲基苯乙胺
N-(p-羟基苯乙基)-n-(2-溴-5-羟基-4-甲氧基苄基)甲酰胺
N-(4-羟基)苄基雷托巴胺
N-(4-甲氧基苄基)-2-(4-甲氧基苯基)乙胺