盐酸原黄素 | 952-23-8
中文名称
盐酸原黄素
中文别名
——
英文名称
proflavine hydrochloride
英文别名
3,6-diaminoacridine hydrochloride;3,6-diaminoacridine monohydrochloride;acridine-3,6-diamine;hydron;chloride
CAS
952-23-8
化学式
C13H11N3*ClH
mdl
——
分子量
245.711
InChiKey
PBBGTVBGXBUVLT-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:270°C (dec.) (lit.)
-
物理描述:Proflavine hydrochloride is a brown powder. (NTP, 1992)
-
溶解度:5 to 10 mg/mL at 68° F (NTP, 1992)
计算性质
-
辛醇/水分配系数(LogP):2.97
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:64.9
-
氢给体数:3
-
氢受体数:3
安全信息
-
WGK Germany:3
-
海关编码:2933990090
-
危险性防范说明:P305+P351+P338
-
危险性描述:H319
-
储存条件:室温且干燥
SDS
Section 1. Chemical Product and Company Identification
Proflavine HCl Catalog
Common Name/
Number(s).
Trade Name
CAS# 952-23-8
Manufacturer
RTECS AR8700000
SPECTRUM CHEMICAL MFG. CORP.
TSCA TSCA 8(b) inventory: No
products were found.
Commercial Name(s) Not available.
CI# Not available.
Synonym 3,6-Acridinediamine hydrochloride
IN CASE OF EMERGENCY
Not available.
Chemical Name
Chemical Family Not available. CALL (310) 516-8000
C13H11N3.HCl
Chemical Formula
SPECTRUM CHEMICAL MFG. CORP.
Section 2.Composition and Information on Ingredients
Exposure Limits
TWA (mg/m3) STEL (mg/m3) CEIL (mg/m3)
Name CAS # % by Weight
1) Proflavine HCl 952-23-8 100
Toxicological Data Not applicable.
on Ingredients
Section 3. Hazards Identification
Potential Acute Health Effects Hazardous in case of ingestion. Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of
inhalation.
Potential Chronic Health Hazardous in case of ingestion.
Slightly hazardous in case of skin contact (irritant), of eye contact (irritant), of inhalation.
Effects
CARCINOGENIC EFFECTS: Not available.
MUTAGENIC EFFECTS: Not available.
TERATOGENIC EFFECTS: Not available.
DEVELOPMENTAL TOXICITY: Not available.
Proflavine HCl
Section 4. First Aid Measures
Eye Contact No known effect on eye contact, rinse with water for a few minutes.
Skin Contact After contact with skin, wash immediately with plenty of water. Gently and thoroughly wash the contaminated skin
with running water and non-abrasive soap. Be particularly careful to clean folds, crevices, creases and groin.
Cover the irritated skin with an emollient. If irritation persists, seek medical attention. Wash contaminated
clothing before reusing.
Serious Skin Contact Not available.
Inhalation Allow the victim to rest in a well ventilated area. Seek immediate medical attention.
Serious Inhalation Not available.
Ingestion Do not induce vomiting. Loosen tight clothing such as a collar, tie, belt or waistband. If the victim is not
breathing, perform mouth-to-mouth resuscitation. Seek immediate medical attention.
Serious Ingestion Not available.
Section 5. Fire and Explosion Data
Flammability of the Product May be combustible at high temperature.
Auto-Ignition Temperature Not available.
Flash Points Not available.
Flammable Limits Not available.
These products are carbon oxides (CO, CO2), nitrogen oxides (NO, NO2...).
Products of Combustion
Fire Hazards in Presence of Not available.
Various Substances
Explosion Hazards in Presence Risks of explosion of the product in presence of mechanical impact: Not available.
of Various Substances Risks of explosion of the product in presence of static discharge: Not available.
Fire Fighting Media SMALL FIRE: Use DRY chemical powder.
and Instructions LARGE FIRE: Use water spray, fog or foam. Do not use water jet.
Special Remarks on Not available.
Fire Hazards
Special Remarks on Explosion Not available.
Hazards
Section 6. Accidental Release Measures
Small Spill Use appropriate tools to put the spilled solid in a convenient waste disposal container. Finish cleaning by
spreading water on the contaminated surface and dispose of according to local and regional authority
requirements.
Large Spill
Use a shovel to put the material into a convenient waste disposal container. Finish cleaning by spreading water
on the contaminated surface and allow to evacuate through the sanitary system.
Proflavine HCl
Section 7. Handling and Storage
Precautions Keep away from heat. Keep away from sources of ignition. Empty containers pose a fire risk, evaporate the
residue under a fume hood. Ground all equipment containing material. Do not breathe dust.
Storage Keep container dry. Keep in a cool place. Ground all equipment containing material. Keep container tightly
closed. Keep in a cool, well-ventilated place. Combustible materials should be stored away from extreme heat
and away from strong oxidizing agents.
Section 8. Exposure Controls/Personal Protection
Engineering Controls Use process enclosures, local exhaust ventilation, or other engineering controls to keep airborne levels below
recommended exposure limits. If user operations generate dust, fume or mist, use ventilation to keep exposure to
airborne contaminants below the exposure limit.
Personal Protection Safety glasses. Lab coat. Dust respirator. Be sure to use an approved/certified respirator or equivalent. Gloves.
Personal Protection in Case of Splash goggles. Full suit. Dust respirator. Boots. Gloves. A self contained breathing apparatus should be used
a Large Spill to avoid inhalation of the product. Suggested protective clothing might not be sufficient; consult a specialist
BEFORE handling this product.
Exposure Limits Not available.
Section 9. Physical and Chemical Properties
Physical state and appearance Solid. Not available.
Odor
Taste Not available.
Molecular Weight 245.71 g/mole
Color Not available.
pH (1% soln/water) Not available.
Boiling Point Not available.
Melting Point Not available.
Critical Temperature Not available.
Not available.
Specific Gravity
Vapor Pressure Not applicable.
Not available.
Vapor Density
Volatility Not available.
Not available.
Odor Threshold
Water/Oil Dist. Coeff. Not available.
Ionicity (in Water) Not available.
Not available.
Dispersion Properties
Solubility Not available.
Section 10. Stability and Reactivity Data
Stability The product is stable.
Instability Temperature Not available.
Conditions of Instability Not available.
Incompatibility with various Not available.
substances
Corrosivity Non-corrosive in presence of glass.
Proflavine HCl
Not available.
Special Remarks on
Reactivity
Not available.
Special Remarks on
Corrosivity
Polymerization No.
Section 11. Toxicological Information
Routes of Entry Ingestion.
Toxicity to Animals LD50: Not available.
LC50: Not available.
Chronic Effects on Humans Not available.
Other Toxic Effects on
Hazardous in case of ingestion.
Humans Slightly hazardous in case of skin contact (irritant), of inhalation.
Special Remarks on
Not available.
Toxicity to Animals
Special Remarks on Not available.
Chronic Effects on Humans
Special Remarks on other Not available.
Toxic Effects on Humans
Section 12. Ecological Information
Ecotoxicity Not available.
Not available.
BOD5 and COD
Possibly hazardous short term degradation products are not likely. However, long term degradation products may
Products of Biodegradation
arise.
Toxicity of the Products The products of degradation are more toxic.
of Biodegradation
Special Remarks on the Not available.
Products of Biodegradation
Section 13. Disposal Considerations
Waste Disposal
Section 14. Transport Information
DOT Classification Not a DOT controlled material (United States).
Identification Not applicable.
Not applicable.
Special Provisions for
Transport
DOT (Pictograms)
Proflavine HCl
Section 15. Other Regulatory Information and Pictograms
No products were found.
Federal and State
Regulations
California
Proposition 65
Warnings
Other Regulations Not available..
WHMIS (Canada) Not controlled under WHMIS (Canada).
Other Classifications
DSCL (EEC) This product is not classified according
to the EU regulations.
Health Hazard
HMIS (U.S.A.) 1 National Fire Protection
1 Flammability
1 Association (U.S.A.)
Fire Hazard
1 0 Reactivity
Health
Reactivity
0
Specific hazard
Personal Protection
E
WHMIS (Canada)
(Pictograms)
DSCL (Europe)
(Pictograms)
TDG (Canada)
(Pictograms)
ADR (Europe)
(Pictograms)
Protective Equipment
Gloves.
Lab coat.
Dust respirator. Be sure to use an
approved/certified respirator or
equivalent.
Safety glasses.
SECTION 16 - ADDITIONAL INFORMATION
N/A
反应信息
-
作为反应物:参考文献:名称:CO-CRYSTALS AND SALTS OF CONTRAST AGENTS AND IMAGING摘要:本发明提供对比剂的共晶体和盐,以及使用和制备共晶体和盐的方法。公开号:US20140004051A1
-
作为产物:描述:3,6-双(二甲基氨基)吖啶 在 双氧水 、 硝酸 、 iron(II) sulfate 作用下, 以 水 为溶剂, 生成 N-de-mono-methyl-acridine orange 、 N,N,N'-de-trimethyl-acridine orange 、 N,N'-de-dimethyl-acridine orange 、 N,N-de-dimethyl-acridine orange 、 盐酸原黄素参考文献:名称:Fenton试剂对水溶液中啶橙染料的化学氧化降解摘要:研究了芬顿试剂(Fe 2+和H 2 O 2)降解水溶液中a啶橙(AO)的性能。研究了不同的反应参数,如初始AO浓度,溶液的pH值,亚铁浓度,过氧化氢浓度和氯离子的存在对AO氧化降解的影响。在最佳条件下,2 mm高2 ö 2,0.4mM的铁2+和pH 3.0时,初始0.2mM的AO溶液减少了95.8%在10分钟内。确定了AO降解反应的主要中间体。分析结果表明,ÑAO染料的去甲基化降解以逐步方式进行,以产生在Fenton过程中生成的单,二,三和四N去甲基化的AO物种。提出并讨论了可能的降解途径。DOI:10.1002/jccs.200900165
文献信息
-
[EN] AZETIDINE-SUBSTITUTED FLUORESCENT COMPOUNDS<br/>[FR] COMPOSÉS FLUORESCENTS À SUBSTITUTION AZÉTIDINE申请人:HUGHES HOWARD MED INST公开号:WO2015153813A1公开(公告)日:2015-10-08The presently-disclosed subject matter includes azetidine-substituted fluorescent compounds, where the compounds may be used as probes, dyes, tags, and the like. The presently-disclosed subject matter also includes kits comprising the same as well as methods for using the same to detect a target substance.目前公开的题材包括取代的荧光化合物,其中化合物可用作探针、染料、标签等。目前公开的题材还包括包含相同成分的试剂盒以及使用相同成分检测目标物质的方法。
-
[EN] PHOTOALIGNING MATERIALS<br/>[FR] MATÉRIAUX DE PHOTOALIGNEMENT申请人:ROLIC AG公开号:WO2013050121A1公开(公告)日:2013-04-11The present invention relates to polymer, homo- or copolymer or oligomer for the photoalignment of liquid crystals, especially for the planar orientation of liquid crystals, comprising a main chain and a side chain, wherein the side chain and/or main chain comprises a polar group, compositions thereof, and its use for optical and electro optical devices, especially liquid crystal devices (LCDs).本发明涉及用于液晶的光调向的聚合物、同聚物或寡聚物,特别用于液晶的平面取向,包括主链和侧链,其中侧链和/或主链包含极性基团,以及其组合物,以及其在光学和电光设备中的应用,特别是液晶设备(LCD)。
-
To Loop or Not to Loop: Influence of Hinge Flexibility on Self-Assembly Outcomes for Acridine-Based Triazolylpyridine Chelates with Zinc(II), Iron(II), and Copper(II)作者:Caitlin E. Miron、Olivier Fleischel、Anne PetitjeanDOI:10.1002/chem.201803732日期:2018.11.22Central to these systems is the need for carefully designed organic ligands, generally with rigid components, that can undergo self‐assembly with metal ions in a predictable manner. Herein, we report the synthesis and study of three novel organic ligands that feature 3,6‐disubstituted acridine as a rigid spacer connected to two 2‐(1,2,3‐triazol‐4‐yl)pyridine “click” chelates through hinges of the same协调驱动的自组装已被确立为有效构建自然和人工系统中复杂结构的有效策略,其应用范围从基因调控到金属有机框架。这些系统的核心是需要精心设计的有机配体,通常带有刚性成分,这些配体可以以可预测的方式与金属离子自组装。本文中,我们报告了三种新型有机配体的合成和研究,这些配体以3,6-二取代的cr啶为刚性间隔基,通过铰链与两个2-(1,2,3-三唑-4-基)吡啶“点击”螯合物连接长度相同,但柔韧性不同。这些“三原子”铰链的灵活性受到以下方面的调节:i)从仲酰胺官能团转移到叔酰胺官能团,以及ii)取代sp2个酰胺碳原子和一个3个亚甲基碳原子。为了理解铰链柔韧性在将自组装引导到单核环或双核圆柱体中的作用,这些变化对锌(II),铁(II)和铜(II)离子对自组装结果的影响是描述。
-
One-step template-directed synthesis of acridine-based rigid cyclophanes作者:Sara Sierra、Katerina Duskova、María-José Fernández、Lourdes Gude、Antonio LorenteDOI:10.1016/j.tet.2012.08.003日期:2012.10Cyclo-bisintercalands are macrocyclic systems containing aromatic subunits, which are commonly used as hosts for aromatic molecules and as DNA intercalators. In this article, a step-by-step synthesis of a series of cyclo-bisintercalands containing 3,6-diaminoacridines as aromatic units, and connected by rigid spacers of different lengths, is reported. In addition, we describe herein a more efficient环-双intercalands是包含芳族亚基的大环系统,通常用作芳族分子的宿主和DNA嵌入剂。在本文中,报告了逐步合成一系列包含3,6-二氨基ac啶作为芳香族单元并通过不同长度的刚性间隔基连接的环双双间隔环的方法。另外,我们在本文中描述了一种更有效的合成替代方案,涉及单步模板指导的过程。新路线允许以可接受的收率容易地合成地进入这些大环系统。合成的双环双间隔环及其前体在结构上已通过紫外可见和NMR方法进行了表征。
-
Studies on the interactions of 3,6-diaminoacridine derivatives with human serum albumin by fluorescence spectroscopy作者:Elmas Gökoğlu、Fulya Kıpçak、Zeynel SeferoğluDOI:10.1002/bio.2635日期:2014.11This study reports the preparation and investigation of the modes of binding of the two symmetric 3,6-diaminoacridine derivatives obtained from proflavine, which are 3,6-diphenoxycarbonyl aminoacridine and 3,6-diethoxycarbonyl aminoacridine to human serum albumin (HSA). The interaction of HSA with the derivatives was investigated using fluorescence quenching and ultraviolet-visible absorption spectra at pH 7.2 and different temperatures. The results suggest that the derivatives used can interact strongly with HSA and are the formation of HSA-derivative complexes and hydrophobic interactions as the predominant intermolecular forces in stabilizing for each complex. The Stern-Volmer quenching constants, binding constants, binding sites and corresponding thermodynamic parameters ΔH, ΔS and ΔG were calculated at different temperatures. The binding distance (r) ~ 3 nm between the donor (HSA) and acceptors (3,6-diethoxycarbonyl aminoacridine, 3,6-diphenoxycarbonyl aminoacridine and proflavine) was obtained according to Förster's non-radiative energy transfer theory. Moreover, the limit of detection and limit of quantification of derivatives were calculated in the presence of albumin. Copyright © 2014 John Wiley & Sons, Ltd.本研究报告了从丙黄嘌呤中得到的两种对称的 3,6-二氨基吖啶衍生物(3,6-二苯氧羰基氨基吖啶和 3,6-二乙氧羰基氨基吖啶)的制备过程以及它们与人血清白蛋白(HSA)的结合模式。在 pH 值为 7.2 和不同温度下,利用荧光淬灭和紫外可见吸收光谱研究了 HSA 与这些衍生物的相互作用。结果表明,所使用的衍生物能与 HSA 发生强烈的相互作用,并形成 HSA 衍生物复合物,疏水相互作用是稳定每种复合物的主要分子间作用力。计算了不同温度下的 Stern-Volmer 淬火常数、结合常数、结合位点和相应的热力学参数 ΔH、ΔS 和 ΔG。根据佛斯特非辐射能量转移理论,得到了供体(HSA)与受体(3,6-二乙氧羰基氨基吖啶、3,6-二苯氧羰基氨基吖啶和丙黄嘌呤)之间的结合距离(r)~3 nm。此外,还计算了在白蛋白存在下衍生物的检出限和定量限。Copyright © 2014 John Wiley & Sons, Ltd. All Rights Reserved.
表征谱图
-
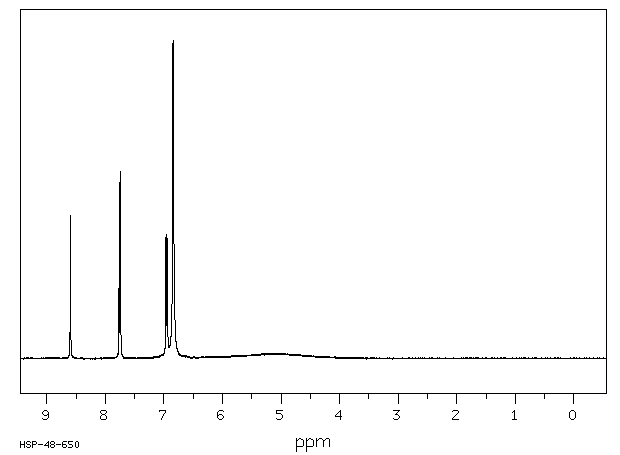
氢谱1HNMR
-
质谱MS
-
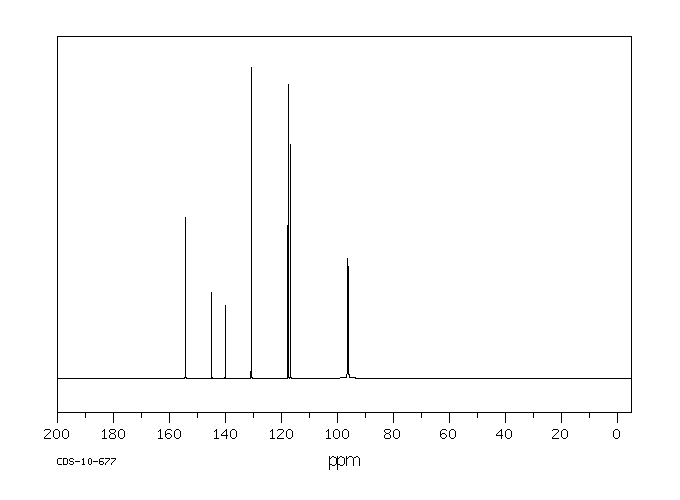
碳谱13CNMR
-
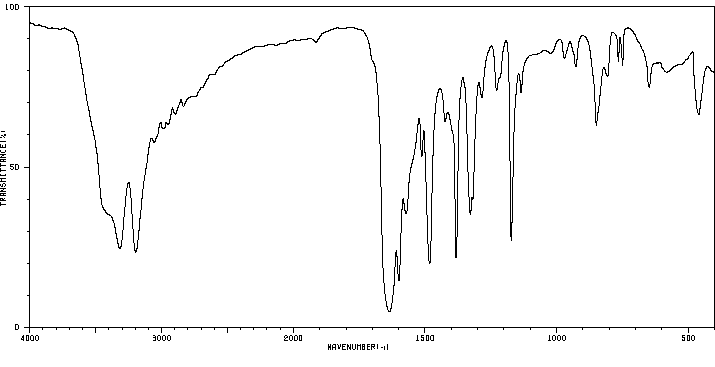
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43