硫氰酸辛酯 | 19942-78-0
中文名称
硫氰酸辛酯
中文别名
——
英文名称
octyl thiocyanate
英文别名
1-thiocyanatooctane;1-octyl thiocyanate;n-octyl thiocyanate
CAS
19942-78-0
化学式
C9H17NS
mdl
MFCD00039477
分子量
171.307
InChiKey
FGVYCHQRRXDRAR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:21℃
-
沸点:170℃
-
密度:0.8625
-
闪点:184℃
计算性质
-
辛醇/水分配系数(LogP):4.3
-
重原子数:11
-
可旋转键数:7
-
环数:0.0
-
sp3杂化的碳原子比例:0.888
-
拓扑面积:49.1
-
氢给体数:0
-
氢受体数:2
SDS
制备方法与用途
化学性质:在常温下,它是一种无色、略带混浊的液态物质。
上下游信息
反应信息
-
作为反应物:参考文献:名称:水对 SmI2 还原的影响:通过 SmI2 促进的硫代硫酸钠和烷基硫氰酸酯的还原合成烷基硫醇的新方法摘要:摘要 在烷基硫代硫酸钠和烷基硫氰酸盐的还原中,水作为助溶剂对SmI2的还原性有显着的提高作用。开发了SmI2/THF/H2O体系合成烷基硫醇的新方法。DOI:10.1081/scc-200028622
-
作为产物:描述:参考文献:名称:Loris, Alessandro; Perosa, Alvise; Selva, Maurizio, Journal of Organic Chemistry, 2003, vol. 68, # 10, p. 4046 - 4051摘要:DOI:
文献信息
-
FLUOROALKYLATING AGENT申请人:IHARA CHEMICAL INDUSTRY CO., LTD.公开号:US20170197920A1公开(公告)日:2017-07-13Problem to be Solved It is intended to provide an industrially preferable fluoroalkylating agent and use thereof. Solution The present invention provides a fluoroalkylating agent represented by the general formula (1) wherein R 1 is a C1 to C8 fluoroalkyl group; R 2 and R 3 are each independently a C1 to C12 alkyl group or the like; Y 1 to Y 4 are each independently a hydrogen atom, a halogen atom, or the like; and X − is a monovalent anion. A compound of the general formula (3): R 4 —S—R 1 having an introduced C1 to C8 fluoroalkyl group is easily obtained by reacting a compound of the general formula (2): R 4 —S—Z wherein R 4 is a hydrocarbon group or the like; and Z is a leaving group, with the compound of the general formula (1).要解决的问题 旨在提供一种工业上可取的氟烷基化剂及其使用方法。 解决方案 本发明提供了一种由通式(1)表示的氟烷基化剂,其中R 1 是C1到C8的氟烷基团;R 2 和R 3 分别独立地是C1到C12的烷基团或类似物;Y 1 到Y 4 分别独立地是氢原子、卤素原子或类似物;X − 是一价阴离子。 通式(3)的化合物:R 4 —S—R 1 ,其中引入了C1到C8的氟烷基团,可通过将通式(2)的化合物:R 4 —S—Z(其中R 4 是烃基团或类似物;Z是离去基团)与通式(1)的化合物反应而轻松获得。
-
Synthesis of (benzenesulfonyl)difluoromethyl thioethers from ((difluoromethyl)sulfonyl)benzene and organothiocyanates generated <i>in situ</i>作者:Cheng Wu、Xiao Shen、Jianjun Dai、Jun Xu、Huajian XuDOI:10.1039/d1ob01215k日期:——available aryl and alkyl thiocyanates were converted into the corresponding (benzenesulfonyl)difluoromethyl thioethers via the direct nucleophilic substitution of ((difluoromethyl)-sulfonyl)benzene under transition metal free conditions. Combined with various thiocyanation methods, this reaction can allow late-stage (benzenesulfonyl)difluoromethylthiolation of alkyl halides and aryl diazonium salts.
-
2-Chloro-1-methylpyridinium iodide, an efficient reagent for the conversion of alcohols into alkyl thiocyanates both under solvent and solvent-free conditions作者:Babak Mokhtari、Roya Azadi、Edris MardaniDOI:10.1016/j.tetlet.2011.11.050日期:2012.2reagent for the simple phosphine-free conversion of alcohols into the corresponding alkyl thiocyanates is described. This transformation can be achieved either in acetonitrile or under solvent-free conditions and the products obtained in good to excellent yields. The solvent-free procedure described here is the first report on the solvent-free thiocyanation of alcohols.
-
Novel compositions申请人:Sandoz Ltd.公开号:US04877899A1公开(公告)日:1989-10-31Sulfur-substituted alkenyl compounds, synthesis thereof, intermediates therefor, and the use of the compounds to control pests.硫化取代的烯基化合物,其合成方法,其中间体,以及这些化合物用于控制害虫的使用。
-
β-Cyclodextrin Immobilized onto Dowex Resin: A Unique Microvessel and Heterogeneous Catalyst in Nucleophilic Substitution Reactions作者:Ali Reza Kiasat、Nasrollah Zarinderakht、Soheil SayyahiDOI:10.1002/cjoc.201280003日期:2012.3The catalytic activity of β‐cyclodextrin immobilized on Dowex resin as an efficient solid‐liquid phase transfer catalyst was developed for the synthesis of alkyl thiocyanates and phenacyl derivatives in water. The nucleophilic substitution reactions were performed under mild reaction condition and gave the products in excellent yields. Furthermore, the catalyst could be recycled by facile separation
表征谱图
-
氢谱1HNMR
-
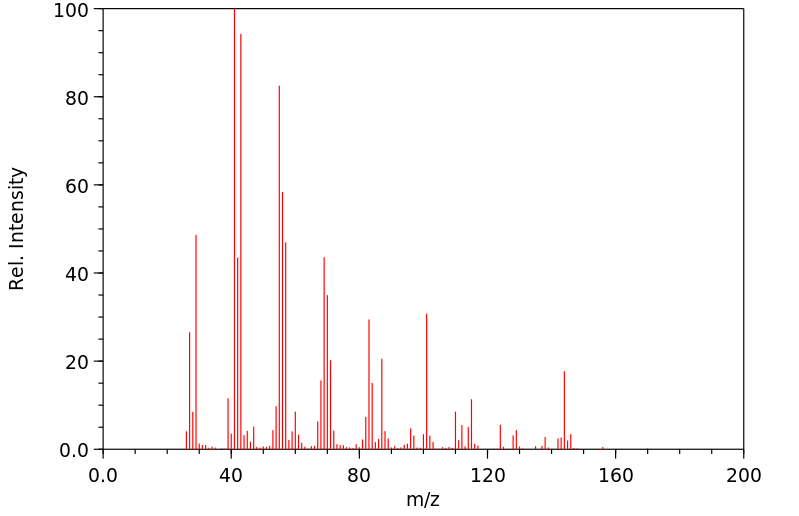
质谱MS
-
碳谱13CNMR
-
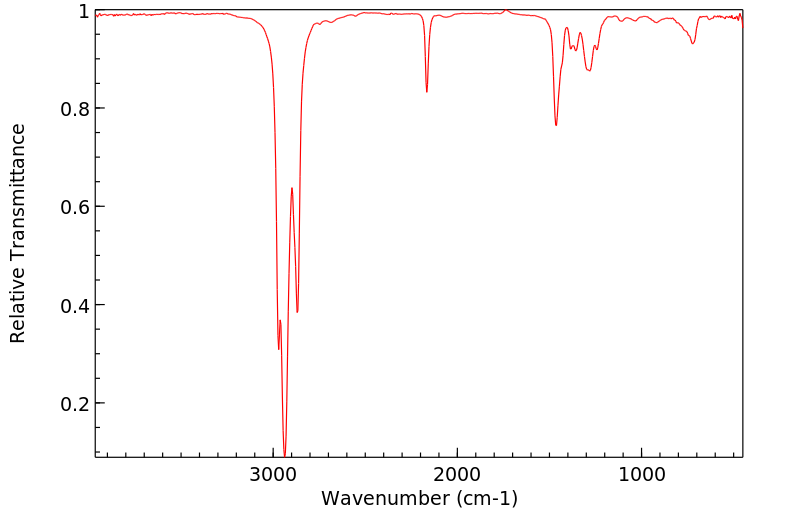
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
酸性橙G
羟基硫氰酸盐
硫氰酸锂二水合物
硫氰酸铵-D4
硫氰酸铵
硫氰酸钡水合物
硫氰酸钠
硫氰酸酯
硫氰酸辛酯
硫氰酸胍
硫氰酸磷酸二乙酯
硫氰酸甲酯
硫氰酸氯甲酯
硫氰酸正十二烷基铵
硫氰酸戊酯
硫氰酸异丙酯
硫氰酸己酯
硫氰酸乙酯
硫氰酸丙酯
硫氰酸丁酯
硫氰酸(三甲硅基甲酯)
硫氰酸 6-氟己基酯
硫氰酸 5-氟戊基酯
硫氰酸 3-氟丙基酯
硫氰酸 2-氟乙基酯
硫氰酸
氰硫基酸氰基甲酯
氯乙烯二硫氰酸酯
正十二烷基硫代异氰酸酯
次硫氰酸根
庚基硫氰酸酯
四亚甲基二硫氰酸酯
叔丁基铵硫氰酸盐
二氰基硫醚
三丁基硫氰酸基锡烷
6-溴-2-(5-甲基噻吩-2-基)喹啉-4-羧酸
2-甲基丙酯硫氰酸酯
2-甲基-2-丙基硫氰酸酯
2-溴丙-2-烯-1-基氰硫基酸酯
2-氯乙基硫代氰酸酯
2-(2-丁氧基乙氧基)-硫氰酸乙酯
1-氟-4-硫氰酸基丁烷
1,3-二(硫氰酸基)丙烷
1,2-二硫氰酸亚乙酯
tetrathiocyano argentate (I) (3-)
ammonium thiocyanate
Manganrhodanid
diazanium;platinum(4+);hexathiocyanate
trans-[Cr(NCS)2(en)2]SCN
Dikalium-Quecksilber(II)-tetrarhodanid