4-氟-4'-羟基联苯 | 324-94-7
中文名称
4-氟-4'-羟基联苯
中文别名
4-氟-4’-羟基二苯;4-(4-氟苯基)苯酚;4'-氟联苯-1-醇;4-氟-4"-羟基联苯
英文名称
4'-fluoro-biphenyl-4-ol
英文别名
4'-fluoro-4-hydroxybiphenyl;4'-fluoro-[1,1'-biphenyl]-4-ol;4-hydroxy-4'-fluorobiphenyl;4-(4-fluorophenyl)phenol;4-(4-Fluor-phenyl)-phenol;4-(p-Fluorphenyl)-phenol;4-Fluoro-4'-hydroxybiphenyl
CAS
324-94-7
化学式
C12H9FO
mdl
——
分子量
188.201
InChiKey
QSJNKJGPJVOGPK-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:152°C
-
沸点:303.7±17.0 °C(Predicted)
-
密度:1.196±0.06 g/cm3(Predicted)
-
溶解度:溶于甲醇
计算性质
-
辛醇/水分配系数(LogP):3.3
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:2
安全信息
-
危险品标志:Xn
-
海关编码:2907199090
-
安全说明:S22,S26,S36/37/39,S45
-
危险类别码:R20/21/22,R37/38,R41,R48
-
危险品运输编号:UN 1090 3/PG 2
-
危险性防范说明:P305+P351+P338
-
危险性描述:H315,H319
-
储存条件:室温
SDS
4-Fluoro-4'-hydroxybiphenyl
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Fluoro-4'-hydroxybiphenyl
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Fluoro-4'-hydroxybiphenyl
Percent: >98.0%(GC)
CAS Number: 324-94-7
Synonyms: 4'-Fluorobiphenyl-1-ol , 4-(4-Fluorophenyl)phenol
C12H9FO
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
4-Fluoro-4'-hydroxybiphenyl
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Very pale yellow
No data available
Odour:
4-Fluoro-4'-hydroxybiphenyl
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:172°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
4-Fluoro-4'-hydroxybiphenyl
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. IDENTIFICATION
Product name: 4-Fluoro-4'-hydroxybiphenyl
Section 2. HAZARDS IDENTIFICATION
GHS classification
PHYSICAL HAZARDS Not classified
HEALTH HAZARDS
Skin corrosion/irritation Category 2
Category 2A
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements, including precautionary statements
Pictograms or hazard symbols
Signal word Warning
Hazard statements Causes skin irritation
Causes serious eye irritation
Precautionary statements:
Wash hands thoroughly after handling.
[Prevention]
Wear protective gloves/eye protection/face protection.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
[Response]
if present and easy to do. Continue rinsing.
If eye irritation persists: Get medical advice/attention.
IF ON SKIN: Gently wash with plenty of soap and water.
If skin irritation occurs: Get medical advice/attention.
Take off contaminated clothing and wash before reuse.
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance/mixture: Substance
Components: 4-Fluoro-4'-hydroxybiphenyl
Percent: >98.0%(GC)
CAS Number: 324-94-7
Synonyms: 4'-Fluorobiphenyl-1-ol , 4-(4-Fluorophenyl)phenol
C12H9FO
Chemical Formula:
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Get medical advice/attention if you feel unwell.
4-Fluoro-4'-hydroxybiphenyl
Section 4. FIRST AID MEASURES
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. If skin irritation or rash occurs: Get medical advice/attention.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing. If eye irritation persists: Get medical
advice/attention.
Ingestion: Get medical advice/attention if you feel unwell. Rinse mouth.
A rescuer should wear personal protective equipment, such as rubber gloves and air-
Protection of first-aiders:
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards arising Take care as it may decompose upon combustion or in high temperatures to
from the chemical: generate poisonous fume.
Precautions for firefighters: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Personal precautions, Use personal protective equipment. Keep people away from and upwind of spill/leak.
protective equipment and Entry to non-involved personnel should be controlled around the leakage area by
emergency procedures: roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Precautions for safe handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a local exhaust if dust or aerosol will be generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Conditions for safe storage, including any
incompatibilities
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Comply with laws.
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust as possible so that workers should not be
exposed directly. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator. Follow local and national regulations.
Hand protection: Protective gloves.
Safety glasses. A face-shield, if the situation requires.
Eye protection:
Skin and body protection: Protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Crystal- Powder
Form:
Colour: White - Very pale yellow
No data available
Odour:
4-Fluoro-4'-hydroxybiphenyl
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
pH: No data available
Melting point/freezing point:172°C
Boiling point/range: No data available
Flash point: No data available
Flammability or explosive
limits:
Lower: No data available
Upper: No data available
Relative density: No data available
Solubility(ies):
[Water] No data available
[Other solvents]
Soluble: Methanol
Section 10. STABILITY AND REACTIVITY
Chemical stability: Stable under proper conditions.
Possibility of hazardous No special reactivity has been reported.
reactions:
Incompatible materials: Oxidizing agents
Hazardous decomposition Carbon monoxide, Carbon dioxide, Hydrogen fluoride
products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
NTP = No data available
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
No data available
Algae:
Persistence / degradability: No data available
No data available
Bioaccumulative
potential(BCF):
Mobility in soil
Log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: Does not correspond to the classification standard of the United Nations
UN-No: Not listed
4-Fluoro-4'-hydroxybiphenyl
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26, 2002
and revised on February 16,2011): Safe use and production, the storage of a dangerous chemical, transport,
loading and unloading were prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
环氧树脂由于具备较高的机械强度、良好的粘接性和加工性能等优点,广泛应用于涂覆材料、模塑料及胶黏剂等领域。然而,其低断裂延伸率和较大的脆性促使研究人员对其进行了广泛的化学或物理改性研究。
4-氟-4'-羟基联苯可以作为原料之一,与1,4-丁二醇、1,6-己二醇、甲苯二异氰酸酯(TDI)、二甲胺及环氧氯丙烷共同合成一种联苯型液晶环氧化合物(LCE)。
制备过程中,在带有搅拌器、温度计和100 mL滴液漏斗的500 mL三颈瓶中,依次加入0.045 mol TMBP(10 g)、0.32 mol 吡啶(8 mL)及100 mL丙酮。将0.1 mol ABC(18 g)溶解在30 mL丙酮中,并通过滴液漏斗缓慢滴加至三颈瓶内。反应在冰水浴中进行约1小时,直至ABC丙酮溶液完全滴加完毕,然后继续在室温下反应8小时。
反应后,向混合物中加入过量的冰水以形成大量沉淀。过滤得到固体,并用氯仿/水(体积比1:1)溶剂洗涤并重结晶。最后,在真空烘箱中干燥后获得黄色粉末状产物。通过FT-IR和NMR光谱对该化合物进行结构表征,确认其为BABTMBP(P1)。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-(4-fluorophenyl)anisole 450-39-5 C13H11FO 202.228 —— 1-(t-Butyldimethylsilyloxy)-4-(4'-fluorophenyl)benzene 135136-44-6 C18H23FOSi 302.464 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 4'-fluoro-[1,1'-biphenyl]-4-yl formate —— C13H9FO2 216.212 对羟基联苯 4-Phenylphenol 92-69-3 C12H10O 170.211 —— 1-Fluoro-4-(4-prop-2-enoxyphenyl)benzene 121721-95-7 C15H13FO 228.266 —— 4-Fluor-p-terphenyl 3799-84-6 C18H13F 248.3 —— [4-(4-Fluorophenyl)phenyl] dihydrogen phosphate 949147-23-3 C12H10FO4P 268.181 —— 3-bromo-4'-fluoro-[1,1'-biphenyl]-4-ol 35770-89-9 C12H8BrFO 267.097 4-氟-4-甲基-1,1-联苯 4-methyl-4'-fluorobiphenyl 72093-43-7 C13H11F 186.229 —— 1,1'-biphenyl-4-yl formate 235416-44-1 C13H10O2 198.221 —— 2-[4-(4-Fluorophenyl)phenoxy]propanoic acid 1764-60-9 C15H13FO3 260.265 4-(4-氟苯基)苯腈 4-cyano-4'-fluorobiphenyl 10540-31-5 C13H8FN 197.212 4-二氟甲氧基联苯 4-(difluoromethoxy)-1,1'-biphenyl 175838-98-9 C13H10F2O 220.219 - 1
- 2
反应信息
-
作为反应物:描述:参考文献:名称:光诱导、苯肼促进的芳基氟化物、氯化物、溴化物和碘化物的无过渡金属脱卤摘要:在这项研究中,我们提出了一种直接且高效的芳基卤化物光触发氢化方法,无需过渡金属催化剂。通过光、PhNHNH2 和碱的协同利用,我们成功地引发了所需的自由基介导的氢化过程。值得注意的是,利用温和的反应条件,多种芳基卤化物,包括氟化物、氯化物、溴化物和碘化物,可以选择性地转化为其相应的(杂)芳烃对应物,且产率极高。此外,该方法表现出与不同官能团和杂环化合物的显着相容性,突出了其多功能性和在各种化学转化中使用的潜力。DOI:10.3390/molecules28196915
-
作为产物:描述:4-(叔丁基二甲基硅氧基)苯基硼酸 在 四(三苯基膦)钯 四丁基氟化铵 、 sodium carbonate 作用下, 以 四氢呋喃 、 水 、 甲苯 为溶剂, 反应 2.08h, 生成 4-氟-4'-羟基联苯参考文献:名称:Discovery of Potent Nonpeptide Inhibitors of Stromelysin Using SAR by NMR摘要:With the use of an NMR-based method, potent (IC50 < 25 nM) nonpeptide inhibitors of the matrix metalloproteinase stromelysin (MMP-3) were discovered. The method, called SAR by NMR (for structure-activity relationships by nuclear magnetic resonance), involves the identification, optimization, and linking of compounds that bind to proximal sites on a protein. Using this technique, two ligands that bind weakly to stromelysin (acetohydroxamic acid, K-D = 17 mM; 3-(cyanomethyl)-4'-hydroxybiphenyl, K-D = 0.02 mM) were identified. On the basis of NMR-derived structural information, the two fragments were connected to produce a 15 nM inhibitor of this enzyme. This compound was rapidly discovered (less than 6 months) and required only a minimal amount of chemical synthesis. These studies indicate that the SAR by NMR method can be effectively applied to enzymes to yield potent lead inhibitors-an important part of the drug discovery process.DOI:10.1021/ja9702778
文献信息
-
Cross-coupling reactions with boronic acids in water catalysed by oxime-derived palladacycles作者:Luis Botella、Carmen NájeraDOI:10.1016/s0022-328x(02)01727-8日期:2002.12Palladacycles derived from phenone-oximes 1 are efficient precatalysts for the Suzuki–Miyaura coupling of arylboronic acids with aromatic and heteroaromatic bromides and chlorides under water reflux under aerobic conditions. Alternatively, the coupling can also be carried out at room temperature in methanol–water. Aryl bromides gave biaryls with TON up to 105 and TOF up to 7×104 h−1. Activated and
-
Reverse hydroxamate inhibitors of matrix metalloproteinases申请人:Abbott Laboratories公开号:US06294573B1公开(公告)日:2001-09-25Compounds having the formula are matrix metalloproteinase inhibitors. Also disclosed are matrix metalloproteinase-inhibiting compositions and methods of inhibiting matrix metalloproteinase in a mammal.
-
Bis(imino)pyridine palladium(II) complexes as efficient catalysts for the SuzukiâMiyaura reaction in water作者:Ping Liu、Mei Yan、Ren HeDOI:10.1002/aoc.1591日期:——Bis(imino)pyridine palladium(II) complexes 3 and 4 of type [PdCl(L)PF6] are found to be efficient catalysts for Suzuki–Miyaura reactions of aryl halides and arylboronic acids. The reactions proceed smoothly to generate the corresponding biaryl compounds in moderate to excellent yields. The synthesis of various fluorinated biphenyl derivatives was successfully achieved by the complex 4 catalyzed the Suzuki–Miyaura
-
PEG Click-Triazole Palladacycle: An Efficient Precatalyst for Palladium-Catalyzed Suzuki-Miyaura and Copper-free Sonogashira Reactions in Neat Water作者:Guofu Zhang、Wei Zhang、Yuxin Luan、Xingwang Han、Chengrong DingDOI:10.1002/cjoc.201500167日期:2015.7A novel water‐soluble, phosphine‐free PEG "click" triazole palladacycle has been successfully synthesized. As a precatalyst, the palladacycle exhibited superior catalytic activity towards Suzuki‐Miyaura and copper‐free Sonogashira cross‐coupling in neat water with the turnover numbers (TONs) of up to 9.8×105. In addition, the catalyst could be reused at least 3 times without significant loss of reactivity
-
A convenient chemical-microbial method for developing fluorinated pharmaceuticals作者:Tara V. Bright、Fay Dalton、Victoria L. Elder、Cormac D. Murphy、Neil K. O'Connor、Graham SandfordDOI:10.1039/c2ob27140k日期:——A significant proportion of pharmaceuticals are fluorinated and selecting the site of fluorine incorporation can be an important beneficial part a drug development process. Here we describe initial experiments aimed at the development of a general method of selecting optimum sites on pro-drug molecules for fluorination, so that metabolic stability may be improved. Several model biphenyl derivatives were transformed by the fungus Cunninghamella elegans and the bacterium Streptomyces griseus, both of which contain cytochromes P450 that mimic oxidation processes in vivo, so that the site of oxidation could be determined. Subsequently, fluorinated biphenyl derivatives were synthesised using appropriate Suzuki–Miyaura coupling reactions, positioning the fluorine atom at the pre-determined site of microbial oxidation; the fluorinated biphenyl derivatives were incubated with the microorganisms and the degree of oxidation assessed. Biphenyl-4-carboxylic acid was transformed completely to 4′-hydroxybiphenyl-4-carboxylic acid by C. elegans but, in contrast, the 4′-fluoro-analogue remained untransformed exemplifying the microbial oxidation – chemical fluorination concept. 2′-Fluoro- and 3′-fluoro-biphenyl-4-carboxylic acid were also transformed, but more slowly than the non-fluorinated biphenyl carboxylic acid derivative. Thus, it is possible to design compounds in an iterative fashion with a longer metabolic half-life by identifying the sites that are most easily oxidised by in vitro methods and subsequent fluorination without recourse to extensive animal studies.大部分药物含有氟元素,在药物开发过程中,选择氟元素的加入位置可能是一个有益的环节。在这里,我们描述了初始实验,旨在开发一种通用方法,选择前药分子上最佳的氟化位置,以提高代谢稳定性。通过含有细胞色素P450的真菌和细菌分别对几种联苯衍生物进行转化,这些细胞色素P450模拟了体内的氧化过程,从而确定了氧化位置。随后,利用适当的铃木-宫浦偶联反应合成了氟化联苯衍生物,将氟原子置于预定微生物氧化的位置;这些氟化联苯衍生物与微生物共培养,并评估了氧化程度。结果显示,联苯-4-羧酸被Cunninghamella elegans完全转化为4′-羟基联苯-4-羧酸,但4′-氟代衍生物保持不变,证明了微生物氧化-化学氟化的概念。2′-氟代和3′-氟代联苯-4-羧酸也能被转化,但速度比未氟化的联苯羧酸衍生物慢。因此,可以通过体外方法确定最容易氧化的位置,然后进行氟化,迭代设计代谢半衰期更长的化合物,无需依赖大量的动物研究。
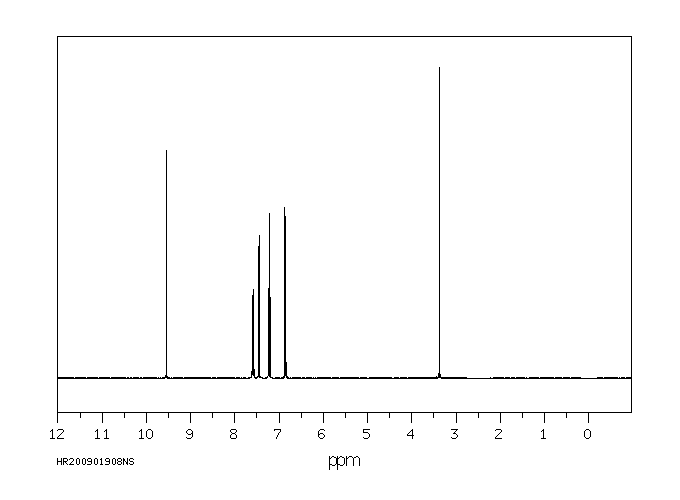
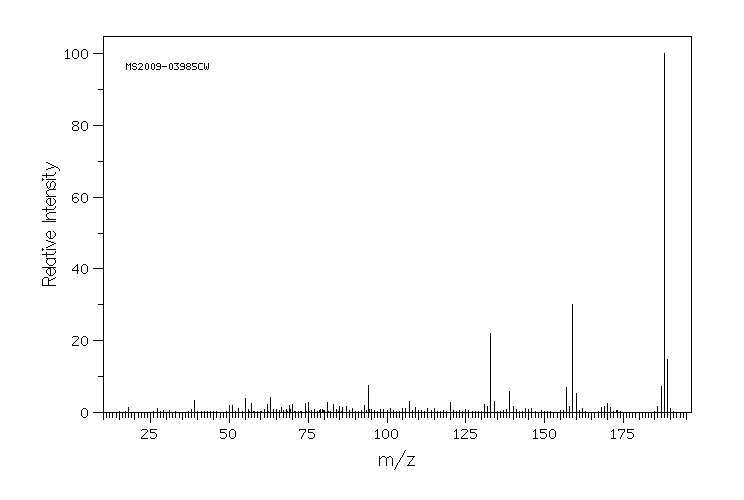
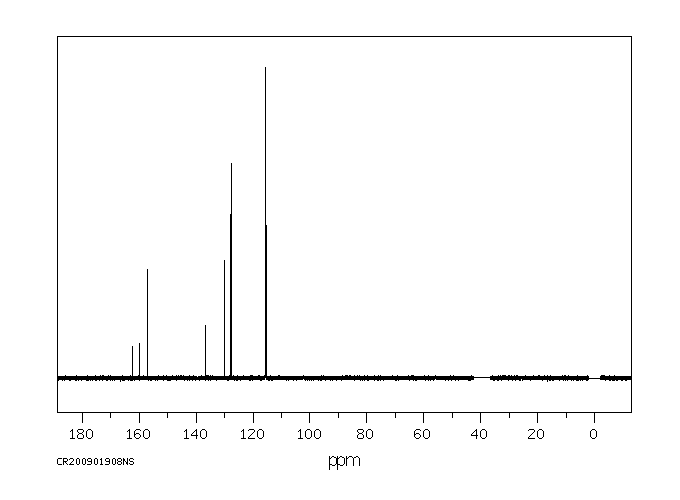
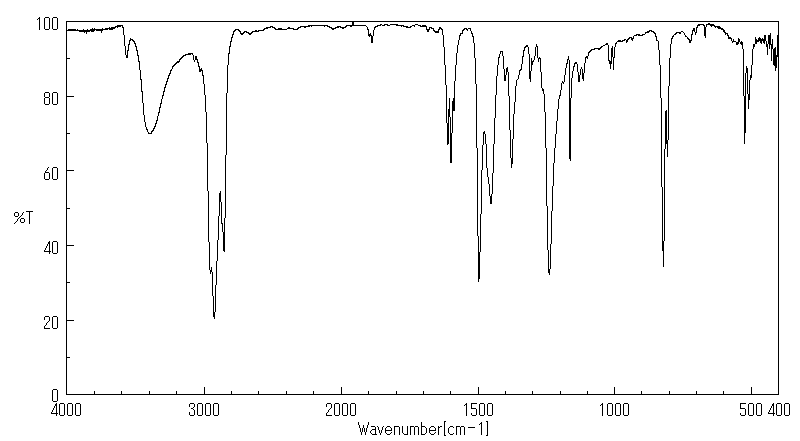
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫