磺胺二甲嘧啶 | 57-68-1
中文名称
磺胺二甲嘧啶
中文别名
2-(对氨基苯磺酰胺基)-4,6-二甲基嘧啶;磺胺间二甲嘧啶;磺胺二甲基嘧啶;4-氨基-N-(4,6-二甲基-2-嘧啶基)苯磺酰胺;N-(4,6-二甲基-2-嘧啶基)-4-氨基苯磺酰胺;SM2
英文名称
sulfadimesine
英文别名
Sulfamethazine;sulfamethazin;sulfadimidine;SMZ;4-amino-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide
CAS
57-68-1
化学式
C12H14N4O2S
mdl
MFCD00006066
分子量
278.335
InChiKey
ASWVTGNCAZCNNR-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:197 °C
-
沸点:294°C (rough estimate)
-
密度:1.2997 (rough estimate)
-
溶解度:丙酮:可溶50mg/mL
-
物理描述:Sulfamethazine appears as odorless sticky, white or creamy-white crystalline powder. Slightly bitter taste. An antibacterial.
-
颜色/状态:Crystals from dioxane-water
-
气味:Odorless
-
味道:Slightly bitter taste
-
蒸汽压力:6.82X10-9 mm Hg at 25 °C (est)
-
稳定性/保质期:
Stable under recommended storage conditions.
-
解离常数:pKa1 = 2.65 (aromatic amine); pKa2 = 7.65 (sulfonamide nitrogen)
-
碰撞截面:161.7 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards]
-
保留指数:2613
计算性质
-
辛醇/水分配系数(LogP):0.3
-
重原子数:19
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.17
-
拓扑面积:106
-
氢给体数:2
-
氢受体数:6
ADMET
代谢
磺胺二甲嘧啶(SDM)及其代谢物在产蛋鸡体内的血浆处置进行了研究,在单次静脉注射、单次口服和连续五天每天一次的多次口服100 mg SDM kg-1剂量后。SDM通过乙酰化和羟基化被广泛代谢。在血浆中,浓度最高的代谢物是N4-乙酰磺胺二甲嘧啶(N4-SDM),其次是羟甲基磺胺二甲嘧啶(CH2OH)和5-羟基磺胺二甲嘧啶。静脉给药后,SDM及其代谢物表现出双相消除(如容量限制反应)模式。多次(5倍)SDM给药显示血浆SDM浓度在7到108微克毫升-1之间;在多次SDM给药终止后的96小时内,血浆SDM浓度低于0.01微克毫升-1。N4-SDM和羟基代谢物的肾清除率大约是SDM的10倍。SDM的质量平衡(粪便/尿液回收)显示,静脉给药后损失了56%,单次口服给药后损失了67%;粪便/尿液中羟基代谢物的百分比最高。因此,在产蛋鸡中必须存在额外的代谢途径。
Plasma disposition of sulfadimidine (SDM) and its metabolites was studied in laying hens after 100 mg SDM kg-1 doses were administered as a single intravenous dose, a single oral dose and multiple oral doses once daily for five consecutive days. SDM was extensively metabolized by acetylation and hydroxylation. In plasma, the metabolite observed with the highest concentration was N4-acetylsulfadimidine (N4-SDM) followed by hydroxymethylsulfadimidine (CH2OH) and 5-hydroxysulfadimidine. Following intravenous administration a biphasic elimination (as seen for a capacity limited reaction) pattern for SDM and its metabolites was observed. Multiple (5x) SDM dosing revealed plasma SDM concentrations ranging between 7 and 108 ug mL-1; within 96 hours of termination of the multiple SDM dosing, the plasma SDM concentration was below 0.01 ug mL-1. The renal clearances of N4-SDM and the hydroxy metabolites were approximately 10 times greater than that of SDM. The SDM mass balance (fecal/urinary recovery) showed a loss of 56 per cent after intravenous dosage and of 67 per cent after a single oral dosage; the hydroxy metabolites accounted for the highest percentage in feces/urine. Thus additional metabolic pathways must exist in laying hens.
来源:Hazardous Substances Data Bank (HSDB)
代谢
在给予10名男性和2名女性健康志愿者口服磺胺二甲嘧啶剂量为12-17 mg/kg体重后,10-20%的剂量以自由和结合的羟基代谢物形式从尿液中排出,61-81%以N4-乙酰磺胺二甲嘧啶形式排出。其中6人被认为是快速乙酰化者,6人是慢速乙酰化者。快速乙酰化者磺胺二甲嘧啶的血浆浓度-时间曲线呈双相,半衰期分别为1.7和5.4小时,而在慢速乙酰化者中呈单相,半衰期为7.6小时。
After 10 male and two female healthy volunteers were given oral doses of sulfamethazine of 12-17 mg/kg bw, 10-20% of the dose was excreted in the urine as free and conjugated hydroxylated metabolites and 61-81% as N4-acetylsulfamethazine. Six of the individuals were considered to be fast acetylators and six slow acetylators. The plasma concentration-time curve for sulfamethazine in the fast acetylators was biphasic, with half-times of 1.7 and 5.4 hr, respectively, whereas in the slow acetylators it was monophasic, with a half-time of 7.6 hr.
来源:Hazardous Substances Data Bank (HSDB)
代谢
Sulfamethazine is metabolized similarly in animals and humans, with N4-acetylation dominating. A trimodal pattern of sulfamethazine acetylation is seen in humans. Differences in acetylation rates were observed between male and female rats and among females of different strains.
来源:Hazardous Substances Data Bank (HSDB)
代谢
磺胺甲噁唑、磺胺甲恶唑、磺胺嘧啶、磺胺吡啶和磺胺二甲嘧啶在人体内的药代动力学已进行研究。代谢物N4-乙酰磺胺的肾脏清除值比相应的母体化合物高6到20倍。磺胺类药物的肾脏清除率取决于尿流量。已经构建了磺胺类药物的N4-乙酰磺胺浓度-时间曲线,用于血浆和尿液。血浆中N4-乙酰磺胺的百分比-时间曲线是建立乙酰化酶表型的极好工具,而从尿样构建的则用处较小。有证据表明,磺胺二甲嘧啶通过2种不同的同工酶进行代谢,而磺胺嘧啶、磺胺吡啶和磺胺甲恶唑则通过1种乙酰化同工酶进行代谢。磺胺甲噁唑的乙酰化程度非常小。
The pharmacokinetics of sulfamethizole, sulfamethoxazole, sulfadiazine, sulfapyridine and sulfadimidine have been studied in man. Renal clearance values of the metabolite N4-acetylsulphonamide are 6 to 20 times higher than those of the corresponding parent compound. The renal clearance of sulfonamides is dependent on the urine flow. N4-Acetylsulfonamide concentration-time profiles for plasma and urine have been constructed for the sulfonamides. The percentage N4-acetylsulfonamide-time profiles for plasma are excellent tools for establishing the acetylator phenotype, while those constructed from urine samples are less useful. Evidence is obtained that sulfadimidine is metabolically processes by 2 different isoenzymes, while sulfadiazine, sulfapyridine and sulfamethoxazole are processes by 1 acetylating isoenzyme. Sulfamethizole is acetylated to very little extent.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
磺胺二甲嘧啶(SMZ)是一种奶油白色的粉末或晶体。它是一种抗感染剂,用于兽医医学中。磺胺二甲嘧啶用作广谱抗菌药,治疗或预防由敏感生物引起的感染。治疗的感染可能包括肺炎、肠道感染(尤其是球虫病)、软组织感染和尿路感染。
人类暴露和毒性:磺胺二甲嘧啶在人类成纤维细胞培养中未诱导非计划性DNA合成。
动物研究:在大鼠饲料中添加600毫克/千克磺胺嘧啶,部分大鼠在第4周和第8周出现甲状腺增生和局限性肥大,但在第13周未出现。在恢复期后,甲状腺的这些变化完全恢复。在正常大鼠的饲料中添加2400和4800毫克磺胺嘧啶/千克,甲状腺的绝对重量和相对重量显著增加。在切除垂体的大鼠中,相对甲状腺重量倾向于略低于正常对照组,但未发现磺胺嘧啶治疗的影响。在正常磺胺嘧啶处理的大鼠中观察到滤泡细胞肥大和增生。切除垂体的大鼠(无TSH)服用SMZ后甲状腺未发生形态学变化。在体外缺乏TSH的情况下,磺胺二甲嘧啶未增加甲状腺细胞增殖,且在给予磺胺二甲嘧啶的长臂猿中甲状腺的结构/功能未受影响。非人灵长类动物和人类对甲状腺过氧化物酶抑制的抵抗力比啮齿类动物强。雌性小鼠被喂食含300、600、1200、2400或3600毫克/千克磺胺二甲嘧啶的饲料或对照饲料,持续90天。在小鼠中,未观察到与治疗相关的肉眼或光镜下的病变。在高剂量(4800 ppm)组的小鼠2年后甲状腺肿瘤发生率增加,但在较低剂量组未增加。大鼠在饲料中分别给予10、40、600、1200或2400 ppm SMZ,以确定SMZ的毒性和潜在致癌性。在处理后24个月杀死的大鼠中,观察到甲状腺滤泡细胞腺癌的发生率与剂量相关显著增加。在治疗动物中甲状腺的非肿瘤性病变发生率显著高于对照组,这些病变包括滤泡细胞增生、滤泡细胞局部细胞改变和多房性囊肿。视网膜萎缩(雄性)和胰腺腺泡萎缩的发生率也随着SMZ剂量的增加而增加。在几种鸟类物种中,接触SMZ会导致血浆中促性腺激素和催乳素的升高。在喂食1%磺胺二甲嘧啶的小鼠组中,未观察到垂体或生殖器官的与治疗相关的组织病理学效应。
大鼠在妊娠第6-15天通过灌胃给药0、540、680或860毫克磺胺嘧啶/千克体重/天。所有处理组母鼠的体重增加减少,相对肝脏重量增加。在高剂量组,胎儿体重降低,每胎畸形胎儿数量增加。每胎胎儿大体或内脏畸形的发生率随着主要畸形为腭裂、输尿管积水增加,中剂量组的输尿管积水和肾积水发生率也有所升高。磺胺二甲嘧啶在5种沙门氏菌伤寒杆菌株(TA 1535、TA 1537、TA 97、TA 98和TA 100)的沙门氏菌/微粒体预培养试验中进行了致突变性测试,包括有和没有代谢激活的情况。磺胺二甲嘧啶在这些测试中为阴性,测试的最高无效剂量为1000微克/平板(1.0毫克/平板)。
IDENTIFICATION AND USE: Sulfamethazine (SMZ) is a creamy-white powder or crystals. It is anti-infective agent used in veterinary medicine. Sulfamethazine is used as a broad-spectrum antimicrobial to treat or prevent infections caused by susceptible organisms. Infections treated may include pneumonia, intestinal infections (especially coccidia), soft tissue infections and urinary tract infections. HUMAN EXPOSURE AND TOXICITY: Sulfamethazine did not induce unscheduled DNA synthesis in human fibroblasts in culture. ANIMAL STUDIES: In rats fed diets containing 600 mg/kg sulfadimidine, hyperplasia and limited hypertrophy of thyroid were seen in some rats at weeks 4 and 8 but not at week 13. There was complete recovery of the changes noted in the thyroid after the recovery period. Absolute and relative thyroid weights increased significantly in normal rats consuming diets containing 2400 and 4800 mg sulfadimidine/kg feed. In the hypophysectomized rats, relative thyroid weights tended to be slightly less than those of normal controls, but no effects of sulfadimidine treatment were found. Follicular-cell hypertrophy and hyperplasia were observed in normal sulfadimidine-treated rats. Hypophysectomized rats (with no TSH) administered SMZ did not develop morphologic changes in the thyroid. Sulfamethazine did not increase thyroid cell proliferation in vitro in the absence of TSH and there was no effect on thyroid structure/function in cynomolgus monkeys administered sulfamethazine. Nonhuman primates and human beings are known to be more resistant than rodents to the inhibition of thyroperoxidase. Female mice were fed either a control diet or a diet containing 300, 600, 1200, 2400 or 3600 mg/kg sulfamethazine for 90 days. In the mice, no treatment-related lesions were seen grossly or by light microscopy. The incidence of thyroid tumors was increased in both male and female mice after 2 yr in the high-dose (4800 ppm) group but not in the lower-dose groups. Rats were given 10, 40, 600, 1200 or 2400 ppm SMZ in the diet to determine the toxicity and potential carcinogenicity of SMZ. A statistically significant dose-related increase in the incidence of follicular cell adenocarcinomas of the thyroid gland was observed in the animals killed after 24 months. The incidences of non-neoplastic lesions of the thyroid gland in treated animals were significantly higher among treated animals than among controls; these lesions included follicular cell hyperplasia, follicular cell focal cellular change and multilocular cysts. The incidences of retinal atrophy, atrophy of the acinar pancreas (males) also increased with increasing SMZ dose. In several avian species exposure to SMZ results in plasma elevation of gonadotropins and prolactin. No treatment-related histopathological effects were observed in the pituitary or reproductive organs of male or female mice in the group fed 1% sulfamethazine. Rats were dosed by gavage with 0, 540, 680, or 860 mg sulfadimidine/kg bw/day on days 6-15 of gestation. Maternal body-weight gain was decreased and relative liver weight was increased in all treated dams. In the high-dose group, fetuses had decreased body weights and the number of malformed fetuses/litter was increased. The incidence of gross or visceral malformations of fetuses/litter was increased with the predominant malformations being cleft palate, hydroureter, and hydronephrosis. The incidence of hydroureter and hydronephrosis was also elevated in the mid-dose group. Sulfamethazine was tested for mutagenicity in the Salmonella/microsome preincubation assay in 5 Salmonella typhimurium strains (TA 1535, TA 1537, TA 97, TA 98, and TA 100) in the presence and absence of metabolic activation. Sulfamethazine was negative in these tests and the highest ineffective dose tested was 1000 ug/plate (1.0 mg/plate).
来源:Hazardous Substances Data Bank (HSDB)
毒理性
评估:对于磺胺二甲嘧啶在人类中的致癌性,证据不足。对于磺胺二甲嘧啶在实验动物中的致癌性,证据充分。总体评估:磺胺二甲嘧啶的致癌性对人不可分类(第3组)。磺胺二甲嘧啶通过非基因毒性机制在小鼠和大鼠中产生甲状腺肿瘤,该机制涉及抑制甲状腺过氧化物酶,导致甲状腺激素浓度改变和甲状腺刺激激素分泌增加。因此,预计磺胺二甲嘧啶对于暴露于不改变甲状腺激素稳态剂量的人类不具有致癌性。流行病学研究以及实验动物毒理学研究提供了令人信服的证据,表明在响应甲状腺激素失衡时,啮齿动物比人类对甲状腺肿瘤的发展敏感得多。
Evaluation: There is inadequate evidence in humans for the carcinogenicity of sulfamethazine. There is sufficient evidence in experimental animals for the carcinogenicity of sulfamethazine. Overall evaluation: Sulfamethazine is not classifiable as to its carcinogenicity to humans (Group 3). Sulfamethazine produces thyroid tumors in mice and rats by a non-genotoxic mechanism, which involves inhibition of thyroid peroxidase resulting in alterations in thyroid hormone concn and incr secretion of thyroid stimulating hormone. Consequently, sulfamethazine would be expected not to be carcinogenic to humans exposed to doses that do not alter thyroid hormone homeostasis. Evidence from epidemiological studies and from toxicological studies in experimental animals provide compelling evidence that rodents are substantially more sensitive than humans to the development of thyroid tumors in response to thyroid hormone imbalance.
来源:Hazardous Substances Data Bank (HSDB)
毒理性
IARC Carcinogenic Agent:Sulfamethazine
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构(IARC)致癌物分类:第3组:无法归类其对人类致癌性
IARC Carcinogenic Classes:Group 3: Not classifiable as to its carcinogenicity to humans
来源:International Agency for Research on Cancer (IARC)
毒理性
国际癌症研究机构专著:第79卷:(2001年)一些甲状腺激素促泌剂
IARC Monographs:Volume 79: (2001) Some Thyrotropic Agents
来源:International Agency for Research on Cancer (IARC)
吸收、分配和排泄
口服给药后迅速吸收。
Rapidly absorbed following oral administration.
来源:DrugBank
吸收、分配和排泄
磺胺二甲嘧啶(SDM)在12周龄的七只矮小前反刍羔羊中,以及18周龄的反刍阶段,即动物18周大时,通过静脉注射100 mg/kg的药代动力学和代谢进行了研究。与12周龄的动物相比,SDM在18周龄羔羊体内的持久性有所延长:在较老的动物中获得了较低的总体清除率和SDM消除的延长。SDM及其代谢物的肾清除值在两个年龄段是相同的。SDM清除率的降低与较老龄时SDM羟基化显著减少有关。减少的氧化性肝脏代谢可能是由于羔羊的性成熟。
The pharmacokinetics and metabolism of sulfadimidine (SDM) following intravenous administration of 100 mg/kg were studied in seven dwarf preruminant kids at 12 weeks of age, and again at the ruminant stage, when the animals were 18 weeks old. The persistence of SDM in 18-week-old kids was prolonged in comparison to the 12-week-old animals: a lower total body clearance and a prolonged elimination of SDM were obtained in the older animals. The renal clearance values of SDM and its metabolites were the same at both ages. The decrease of SDM clearance is related to the significant reduction in SDM hydroxylation at the older age. The reduced oxidative hepatic metabolism may result from the sexual maturation of the kids.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
磺胺二甲嘧啶乙酰化表型在19名健康成年人(年龄17-46岁;15名男性,4名女性;9名白人,9名东方人,1名黑人)中进行了测定,他们单次口服了20 mg/kg体重的磺胺二甲嘧啶,溶于200毫升水中。结果显示,乙酰化清除率和整体消除或代谢速率常数呈现出明确的三角模式,并确认快速乙酰化表型可以细分为中间型和快速型乙酰化组。快速乙酰化者的平均乙酰化清除率(1.34 mL/min per kg bw)是慢速乙酰化者估计清除率(0.15 mL/min per kg bw)的8.8倍,是中间型乙酰化者(0.75 mL/min per kg bw)的1.8倍。在72小时内,快速乙酰化者、中间型乙酰化者和慢速乙酰化者尿液中以乙酰磺胺二甲嘧啶形式排泄的吸收剂量的百分比平均值分别为93.7、87.7和65.6。
Sulfamethazine acetylation phenotypes were determined in 19 healthy adults (aged 17-46 years; 15 men, four women; nine white, nine oriental, one black) given a single oral dose of 20 mg/kg bw sulfamethazine in 200 mL of water. The results showed a welldefined trimodal pattern for acetylation clearance and for overall elimination or metabolic rate constants and confirmed that the fast acetylator phenotype can be subdivided into intermediate and rapid acetylator groups. The average acetylation clearance rate for rapid acetylators (1.34 mL/min per kg bw) was 8.8 times the estimated clearance for slow acetylators (0.15 mL/min per kg bw) and 1.8 times that for intermediate acetylators (0.75 mL/min per kg bw). The average percentage of an absorbed dose excreted as acetylsulfamethazine in 72-hr urine was 93.7 for rapid acetylators, 87.7 for intermediate acetylators and 65.6 for slow acetylators.
来源:Hazardous Substances Data Bank (HSDB)
吸收、分配和排泄
研究了鸡在单次或多次口服100毫克/千克磺胺二甲嘧啶(SDM)后所产蛋中SDM及其N4-乙酰化和羟基代谢物的耗竭情况。在给药期间以及最后一次给药后一天内,蛋清中的SDM及其代谢物浓度超过了蛋黄,并反映了血浆水平。从给药后两天开始,蛋黄中的SDM浓度变得高于蛋清,药物耗竭曲线平行运行。单次给药后全蛋中SDM的平均最大量为1500微克,多次给药后为1280微克。在蛋中可以检测到N4-乙酰化和6-甲基羟基代谢物的痕迹(主要在蛋清中),它们的浓度大约是母药的40倍以下。卵母细胞在(最后)给药时的发育阶段与从其中发育出的蛋中发现的SDM量之间存在高度显著的相关性(P小于0.005)。为了获得低于0.1微克/克蛋的SDM水平,需要在100毫克/千克/天的(最后)剂量后经过7或8天的时间。
The depletion of sulfadimidine (SDM) and its N4-acetyl and hydroxy metabolites was studied in eggs laid by hens after administration of either a single or multiple oral dosages of 100 mg SDM/kg. During medication and until 1 day after the last dose, the SDM and its metabolite concentrations in the egg white exceeded those in the egg yolk and reflected the plasma levels. In the period starting 2 days after the (last) dosage, the SDM concentration in the yolk became higher than in the egg white, and the drug depletion curves ran parallel. The mean maximum amount of SDM found in the whole egg was 1500 micrograms after a single and 1280 ug after multiple dosage. In eggs, traces of the N4-acetyl and 6-methylhydroxy metabolites could be detected (mainly in the egg white), and their concentrations were approximately 40 times lower than those of the parent drug. A highly significant correlation (P less than 0.005) was found between the development stage of the oocyte at the time of (last) medication and the amount of SDM found in the egg that developed from it. A period of 7 or 8 days after the (last) dosage of 100 mg SDM/kg/day is required to obtain SDM levels below 0.1 ug/g egg.
来源:Hazardous Substances Data Bank (HSDB)
安全信息
-
TSCA:Yes
-
危险品标志:Xi
-
安全说明:S23,S24/25
-
危险类别码:R36/37/38
-
WGK Germany:2
-
海关编码:2935009090
-
危险品运输编号:25kgs
-
RTECS号:WO9275000
-
危险性防范说明:P280,P305+P351+P338
-
危险性描述:H302
-
储存条件:本品应密封存放在阴凉干燥、避光的地方。
SDS
磺胺二甲嘧啶 修改号码:5
模块 1. 化学品
产品名称: Sulfamethazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
生殖毒性 第2级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 怀疑会损害生育能力或胎儿
防范说明
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
使用个人所需的防护用具。
[急救措施] 如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 磺胺二甲嘧啶
百分比: >98.0%(LC)(T)
CAS编码: 57-68-1
俗名: Sulfadimidine , 4-Amino-N-(4,6-dimethyl-2-pyrimidyl)benzenesulfonamide ,
4,6-Dimethylsulfadiazine
分子式: C12H14N4O2S
磺胺二甲嘧啶 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。冷藏储存。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
磺胺二甲嘧啶 修改号码:5
模块 9. 理化特性
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
199°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: orl-mus LD50:50000 mg/kg
scu-rat LD50:2000 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 3 (无法对人类的致癌性进行分类)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: WO9275000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
磺胺二甲嘧啶 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Sulfamethazine
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
生殖毒性 第2级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 怀疑会损害生育能力或胎儿
防范说明
[预防] 使用前获取特定手册。
处理前必须阅读并理解所有安全措施。
使用个人所需的防护用具。
[急救措施] 如接触到或相关接触:求医/就诊。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 磺胺二甲嘧啶
百分比: >98.0%(LC)(T)
CAS编码: 57-68-1
俗名: Sulfadimidine , 4-Amino-N-(4,6-dimethyl-2-pyrimidyl)benzenesulfonamide ,
4,6-Dimethylsulfadiazine
分子式: C12H14N4O2S
磺胺二甲嘧啶 修改号码:5
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。
求医/就诊。
食入: 求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免所有部位的接触!
贮存
储存条件: 保持容器密闭。冷藏储存。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
热敏, 光敏
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
磺胺二甲嘧啶 修改号码:5
模块 9. 理化特性
颜色: 白色类白色
气味: 无资料
pH: 无数据资料
熔点:
199°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 硫氧化物
模块 11. 毒理学信息
急性毒性: orl-mus LD50:50000 mg/kg
scu-rat LD50:2000 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 3 (无法对人类的致癌性进行分类)。
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: WO9275000
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
磺胺二甲嘧啶 修改号码:5
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
作用
磺胺二甲嘧啶是一种广谱抑菌剂。它通过竞争性地作用于细菌体内的对氨基苯甲酸(PABA),阻止其作为原料参与细菌所需叶酸的合成过程,从而减少了具有代谢活性的四氢叶酸的量。后者是细菌合成嘌呤、胸腺嘧啶核苷和脱氧核糖核酸(DNA)所必需的物质。因此,磺胺二甲嘧啶能够抑制细菌的生长繁殖。PABA及其衍生物(如普鲁卡因、丁卡因)、脓液以及组织分解产物的存在可以拮抗其作用。
合成将1.0毫摩尔对乙酰氨基苯磺酰氯和1.5毫摩尔环胺溶解于1毫升水中,搅拌5分钟后,通过过滤收集所得沉淀物。将其转移到圆底烧瓶中,加入2毫升盐酸溶液以及4毫升水。在100°C下轻轻煮沸一小时后,将反应混合物冷却至冰浴,并使用饱和碳酸氢钠水溶液碱化。随后过滤颗粒状沉淀物并用水洗涤,在空气中干燥,最终得到产品磺胺二甲嘧啶。
化学性质磺胺二甲嘧啶为白色或微黄色结晶或粉末,熔点176°C。它几乎不溶于水(29℃溶解度:150毫克/100毫升;37℃溶解度:192毫克/100毫升),但在热乙醇中易溶且不溶于乙醚,易于稀酸或稀碱溶液中溶解。无嗅味微苦,遇光颜色逐渐变深。
用途磺胺二甲嘧啶用于防治葡萄球菌及溶解性链球菌等感染,并对溶血性链球菌、胸膜炎球菌等细菌具有抑制作用,主要用于治疗禽霍乱、禽伤寒和鸡球虫病。作为兽药,它可用于分析检测;作为一种抗菌磺胺药物,它能诱导CYP3A4表达,通过N-乙酰转移酶乙酰化表现出性依赖的药物代谢动力学,并通过特定同源异构体CYP2C11产生代谢变化;此外还具有抑制二氢叶酸合成酶的功能,从而阻碍叶酸合成。它对溶血性链球菌、肺炎球菌及脑膜炎等细菌亦有抑制作用。
生产方法上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 PET胶水 N-[4-(4,6-Dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-acetamide 100-90-3 C14H16N4O3S 320.372 N-(4,6-二甲基-2-嘧啶)-4-硝基苯磺酰胺 N-(4,6-dimethyl-pyrimidin-2-yl)-4-nitro-benzenesulfonamide 153312-38-0 C12H12N4O4S 308.318 —— [4-(4,6-dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-carbamic acid ethyl ester 50910-49-1 C15H18N4O4S 350.398 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— N-(4,6-dimethylpyrimidin-2-yl)-4-isothiocyanatobenzenesulfonamide 51908-34-0 C13H12N4O2S2 320.396 —— N-(4,6-dimethylpyrimidin-2-yl)-4-isocyanatobenzenesulfonamide 1267610-53-6 C13H12N4O3S 304.329 —— N-hydroxymethyl-sulfanilic acid-(4,6-dimethyl-pyrimidin-2-ylamide) —— C13H16N4O3S 308.361 —— N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 6149-31-1 C12H13N3O2S 263.32 PET胶水 N-[4-(4,6-Dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-acetamide 100-90-3 C14H16N4O3S 320.372 5-羟基磺胺二甲嘧啶 5-Hydroxysulphadimidine 51395-19-8 C12H14N4O3S 294.334 —— 4-[(2,2-dicyanoethenyl)amino]-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 910418-04-1 C16H14N6O2S 354.392 —— N4'-chloroacetylsulphamethazine 116488-93-8 C14H15ClN4O3S 354.817 —— sulfamethazine acrylate 59941-96-7 C15H16N4O3S 332.383 —— N-(4,6-dimethyl-2-pyrimidine)-4-N-(benzenesulfonamide)benzenesulfonamide —— C18H19N5O4S2 433.512 —— 4-bromo-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide —— C12H12BrN3O2S 342.216 N-(4,6-二甲基嘧啶-2-基)-4-[(苯基氨基甲硫杂酰)氨基]苯磺酰胺 N-(4,6-dimethyl-pyrimidin-2-yl)-4-(3-phenyl-thioureido)-benzenesulfonamide 17486-59-8 C19H19N5O2S2 413.524 —— N-(4,6-dimethyl-pyrimidin-2-yl)-4-chloro-benzenesulfonamide —— C12H12ClN3O2S 297.765 N-(4,6-二甲基嘧啶-2-基)-4-[2-[4-[(4,6-二甲基嘧啶-2-基)氨基磺酰基]苯基]亚氨基肼基]苯磺酰胺 1,3-di-(4(N-(4,6-dimethyl-2-pyrimidinyl))sulfamoylphenyl)triazene 110505-56-1 C24H25N9O4S2 567.652 —— N-{4-[N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}carbonohydrazonoyl dicyanide 93987-45-2 C15H13N7O2S 355.38 —— 4-benzylamino-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide 491600-29-4 C19H20N4O2S 368.459 —— N-dichloroacetyl-sulfanilic acid-(4,6-dimethyl-pyrimidin-2-ylamide) 107619-10-3 C14H14Cl2N4O3S 389.262 —— 4-((4-(dimethylamino)phenyl)diazenyl)-N-(4,6-dimethylpyrimidin-2-yl)benzene sulfonamide 110675-54-2 C20H22N6O2S 410.5 —— N-(4,6-dimethylpyrimidin-2-yl)-4-((4-(ethylamino)phenyl)diazenyl)benzene sulfonamide 1353055-76-1 C20H22N6O2S 410.5 —— N-[4-[[(4,6-dimethyl-2-pyrimidinyl)amino]sulfonyl]phenyl]pentanamide 101603-55-8 C17H22N4O3S 362.453 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(3-(4-fluorophenyl)ureido)benzenesulfonamide —— C19H18FN5O3S 415.448 —— 2-chloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)propanamide —— C15H17ClN4O3S 368.844 —— methacryloyl sulfamethazine 59941-98-9 C16H18N4O3S 346.41 —— 4-(3-allyl-thioureido)-N-(4,6-dimethyl-pyrimidin-2-yl)-benzenesulfonamide 17486-66-7 C16H19N5O2S2 377.491 —— N-[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]decanamide —— C22H32N4O3S 432.587 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(3-(4-chlorophenyl)ureido)benzenesulfonamide 59440-78-7 C19H18ClN5O3S 431.903 —— N-[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]-3-oxobutanamide —— C16H18N4O4S 362.409 4-({4-[(4,6-二甲基-2-嘧啶基)氨基磺酰基]苯基}氨基)-4-氧代丁酸 N-[4-(4,6-dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-succinamic acid 85828-79-1 C16H18N4O5S 378.409 —— N-[4-(4,6-dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-maleamic acid 37560-05-7 C16H16N4O5S 376.393 —— 4-amino-N-(4,6-dimethyl-2-pyrimidinyl)-N-methylbenzenesulfonamide 63826-13-1 C13H16N4O2S 292.362 —— 4-((4-(diethylamino)phenyl)diazenyl)-N-(4,6-dimethylpyrimidin-2-yl)benzene sulfonamide 1353055-75-0 C22H26N6O2S 438.553 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(3-(3-chlorophenyl)ureido)benzenesulfonamide —— C19H18ClN5O3S 431.903 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-phenylpropanamide —— C21H22N4O3S 410.497 —— N-(4,6-dimethyl-pyrimidin-2-yl)-4-(2-hydroxy-benzylideneamino)-benzenesulfonamide 16182-36-8 C19H18N4O3S 382.443 —— N-(4,6-dimethylpyrimidin-2-yl)-4-[(2-hydroxybenzylidene)amino]benzene sulfonamide 16182-36-8 C19H18N4O3S 382.443 —— N-(4-ethoxy-phenyl)-N'-[4-(4,6-dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-thiourea 103506-97-4 C21H23N5O3S2 457.577 —— Ethyl 4-({4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}amino)-4-oxobutanoate —— C18H22N4O5S 406.462 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide 102017-64-1 C19H18N4O3S 382.443 —— N-[4-(4,6-dimethylpyrimidin-2-sulfamoyl)phenyl]pyrimidine-2-carboximidamide 1429897-29-9 C17H17N7O2S 383.434 —— 4-{[(cyclohexylamino)carbonyl]amino}-N-(4,6-dimethyl-2-pyrimidinyl)benzenesulfonamide —— C19H25N5O3S 403.5 —— 4-(3-(3,4-dichlorophenyl)ureido)-N-(4,6-dimethylpyrimidin-2-yl)benzene-sulfonamide —— C19H17Cl2N5O3S 466.348 —— 4-(Benzhydrylamino)-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide 491600-59-0 C25H24N4O2S 444.557 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(1H-pyrrol-1-yl)benzenesulfonamide —— C16H16N4O2S 328.395 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-4-methylbenzamide 110534-79-7 C20H20N4O3S 396.47 —— 4-chloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H17ClN4O3S 416.888 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(2,5-dioxo-2,5-dihydro-1H-pyrrol-1-yl)benzene sulfonamide 233761-16-5 C16H14N4O4S 358.378 —— ethyl {[4-(N-(4,6-dimethylpyrimidin-2-yl))sulfamoyl]phenylazo}cyanoacetate 1428740-60-6 C17H18N6O4S 402.434 —— N,N-succinyl-sulfanilic acid-(4,6-dimethyl-pyrimidin-2-ylamide) —— C16H16N4O4S 360.393 —— 4-bromo-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide 5251-36-5 C19H17BrN4O3S 461.339 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-4-fluorobenzamide —— C19H17FN4O3S 400.433 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-methylbenzamide —— C20H20N4O3S 396.47 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3,5-dimethylbenzamide —— C21H22N4O3S 410.497 —— 4-[(1H-benzimidazol-2-ylmethyl)-amino]-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide 107090-13-1 C20H20N6O2S 408.484 —— N-(4,6-dimethylpyrimidin-2-yl)-4-((4-oxo-4,5-dihydrothiazol-2-yl)amino)benzenesulfonamide —— C15H15N5O3S2 377.448 —— 3-chloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H17ClN4O3S 416.888 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-iodobenzamide —— C19H17IN4O3S 508.339 —— 4-([(E)-(5-bromo-2-hydroxyphenyl)methylidene]amino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 109556-93-6 C19H17BrN4O3S 461.339 —— N-[4-[[(4,6-dimethyl-2-pyrimidinyl)amino]sulfonyl]phenyl][1,1’-biphenyl]-4-carboxamide —— C25H22N4O3S 458.541 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-2-methylbenzamide —— C20H20N4O3S 396.47 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-fluorobenzamide —— C19H17FN4O3S 400.433 —— 3-bromo-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H17BrN4O3S 461.339 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-4-(trifluoromethyl)benzamide —— C20H17F3N4O3S 450.441 —— N-[4-(4,6-dimethylpyrimidin-2-sulfamoyl)phenyl]pyrazine-2-carboximidamide 1429897-32-4 C17H17N7O2S 383.434 —— N-({4-[N′-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}carbamothioyl)benzamide 313959-07-8 C20H19N5O3S2 441.535 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-4-nitrobenzamide 110148-53-3 C19H17N5O5S 427.44 —— 4-(2,4-dimethoxybenzylamino)-N-(4,6-dimethylpyrimidin-2-yl)-benzenesulfonamide 491600-51-2 C21H24N4O4S 428.512 —— 2,5-dichloro-N-[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]benzenesulfonamide 88522-23-0 C18H16Cl2N4O4S2 487.387 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-2-fluorobenzamide —— C19H17FN4O3S 400.433 —— 4-((4-(benzyl(ethyl)amino)phenyl)diazenyl)-N-(4,6-dimethylpyrimidin-2-yl)benzene sulfonamide 1353055-77-2 C27H28N6O2S 500.624 —— 2-bromo-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H17BrN4O3S 461.339 —— 2-(4-sulfamethazine)hydrazono-5,5-dimethylcyclohexane-1,3-dione 114931-01-0 C20H23N5O4S 429.5 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(phthalazin-1-ylamino)benzenesulfonamide —— C20H18N6O2S 406.468 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3- trifluoromethylbenzamide —— C20H17F3N4O3S 450.441 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-methoxybenzamide 113012-37-6 C20H20N4O4S 412.469 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3,4-difluorobenzamide —— C19H16F2N4O3S 418.424 —— N-(4,6-dimethylpyrimidin-2-yl)-4-{[(2,4,6-trioxotetrahydropyrimidin-5(2H)-ylidene)methyl]amino}benzenesulfonamide 392663-27-3 C17H16N6O5S 416.417 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(1H-tetrazol-1-yl)benzenesulfonamide —— C13H13N7O2S 331.358 —— (3Z)-3-[[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]hydrazinylidene]-6-oxocyclohexa-1,4-diene-1-carboxylic acid 2315-08-4 C19H17N5O5S 427.44 —— 4-fluoro-N-({4-[N′-(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl}carbamothioyl)benzamide 331443-81-3 C20H18FN5O3S2 459.525 —— ethyl 2,3-dioxobutyrate-2-<(N1-2-(4,6-dimethyl)pyrimidyl)sulfonamidophenyl>hydrazone 74731-81-0 C18H21N5O5S 419.461 —— 2,4-dichloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H16Cl2N4O3S 451.333 —— N-(4,6-dimethylpyrimidin-2-yl)-4-{[(4,6-dioxo-2-thioxotetrahydropyrimidin-5(2H)-ylidene)methyl]amino}benzenesulfonamide 1021228-88-5 C17H16N6O4S2 432.484 —— 4-(3-(2-(4-bromophenoxy)acetyl)thioureido)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide —— C21H20BrN5O4S2 550.457 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3,5-dimethoxybenzamide —— C21H22N4O5S 442.495 —— 4-[2-[4-[(R)-(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]ethylamino]-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 1159975-51-5 C31H35ClN6O2S 591.177 —— 2,5-dichloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)benzamide —— C19H16Cl2N4O3S 451.333 —— (E)-4-(2-(3-amino-5-oxo-1H-pyrazol-4(5H)-ylidene)hydrazinyl)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide —— C15H16N8O3S 388.41 —— N-(2-methoxy-benzoyl)-sulfanilic acid-(4,6-dimethyl-pyrimidin-2-ylamide) 112865-29-9 C20H20N4O4S 412.469 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3-nitrobenzamide 110149-75-2 C19H17N5O5S 427.44 —— 4-[3-[4-[(R)-(4-chlorophenyl)-phenylmethyl]piperazin-1-yl]propylamino]-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 1159975-60-6 C32H37ClN6O2S 605.204 —— 4-[(7-chloroquinolin-4-yl)amino]-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide 1449365-40-5 C21H18ClN5O2S 439.925 2-[[[4-[[(4,6-二甲基-2-嘧啶基)氨基]磺酰基]苯基]氨基]羰基]苯甲酸 N-[4-(4,6-dimethyl-pyrimidin-2-ylsulfamoyl)-phenyl]-phthalamic acid 94134-30-2 C20H18N4O5S 426.453 —— N-(4,6-dimethylpyrimidin-2-yl)-4-[2-(1,3-dioxo-1,3-diphenylpropan-2-ylidene)hydrazinyl]benzenesulfonamide —— C27H23N5O4S 513.6 —— 4-[(4-amino-5-oxo-1,2,4-triazin-3-yl)amino]-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide —— C15H16N8O3S 388.41 —— 5-chloro-N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-2-hydroxybenzamide 1369701-22-3 C19H17ClN4O4S 432.887 —— Z-4-(3-(3,4-dimethoxyphenyl)-3-oxoprop-1-enylamino)-N-(4,6-dimethylpyrimidin-2-yl)benzenesulfonamide —— C23H24N4O5S 468.533 —— N-[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]-2-[[2-[[4-[(4,6-dimethylpyrimidin-2-yl)sulfamoyl]phenyl]carbamoyl]phenyl]disulfanyl]benzamide —— C38H34N8O6S4 827.002 —— N-(4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl)-3,5-dinitrobenzamide —— C19H16N6O7S 472.438 —— N-{[4-(N-(4,6-dimethylpyrimidin-2-yl)sulfamoyl)phenyl]carbamoyl}quinoxaline-2-carboxamide —— C21H18N6O3S 434.478 —— N-(4,6-dimethylpyrimidin-2-yl)-4-(7-(trifluoromethyl)quinolin-4-ylamino)benzenesulfonamide 1489236-28-3 C22H18F3N5O2S 473.478 - 1
- 2
- 3
- 4
- 5
- 6
- 7
- 8
- 9
- 10
反应信息
-
作为反应物:参考文献:名称:聚合物配合物:[Sulpha药物衍生物-N,S:N,O]配合物超分子组装中配位π-π堆积和氢键的XLXII相互作用摘要:5-磺胺重氮偶氮-3-苯基氨基-2-硫代-4-噻唑烷酮(HL 1),5-磺胺嘧啶-3-苯基氨基-2-硫代-4-噻唑烷酮(HL 2)的一系列新型镍(II)聚合物配合物,5-硫酸甲恶唑-3-苯基氨基-2-硫代-4-噻唑烷酮(HL 3),5-硫酸乙酰胺-3-苯基-2-硫代-4-噻唑烷酮(HL 4)和5-磺基胍-3-苯基氨基-2制备并表征了-thioxo-4-thiazolidinone(HL 5)。红外光谱表明,HL n(n = 1-5)以中性四齿态与金属离子配位,并带有NH(hydr),NH(3-苯胺),羰基和Ph-NH的NSNO供体。标题[Ni 3(HL n)2(μ-OAc)2(OAc)4 ] n由三个通过链间π-π相互作用连接的Ni(II)原子组成,观察到两个配体(HL n)的芳环之间进一步加成桥连,两个相邻的镍原子通过乙酸酯基。发现这些络合物的几何结构为八面体。结合的性质和配合物的立体化学已从元素分析,热,红外,1DOI:10.1016/j.saa.2010.07.011
-
作为产物:参考文献:名称:CH231195摘要:公开号:
-
作为试剂:描述:甲苯磺丁脲 在 liver microsomes of Caprus hircus aegagrus 、 磺胺二甲嘧啶 作用下, 以 水 为溶剂, 反应 8.0h, 生成 4-Hydroxy tolbutamide参考文献:名称:Differential inhibitory effects of phenytoin, diclofenac, phenylbutazone and a series of sulfonam ides on hepatic cytochrom e P4502C activityin vitro, and correlation with som e m olecular descriptors in the dwarf goat (Caprus hircus aegagrus).摘要:The aim of the present study was to investigate the potency of various sulfonamides to inhibit tolbutamide hydroxylation (a CYP2C activity) in hepatic microsomal fractions and hepatocytes of the dwarf goat. Also a number of suggested substrates for human CYP2C9 was investigated.2. From Dixon plots (microsomal fractions) if was observed that all compounds were competitive inhibitors of tolbutamide hydroxylation. Phenytoin (PT) showed the lowest K-i. K-i for the sulfonamides ranged between 205 and 4546 mu m, sulfadoxine having the lowest K-i followed by sulfadimethoxine, sulfamoxole, sulfadimidine and sulfaphenazole.3. In hepatocytes sulfaphenazole and diclofenac were the most potent inhibitors.4. Out data indicate that PT, diclofenac (DF) and phenylbutazone (PBZ) are relative strong competitive inhibitors of tolbutamide hydroxylation and they are probably also substrates for the same enzyme. Differential inhibition of tolbutamide hydroxylation by sulfonamides was observed.5. Correlation of structural parameters with the inhibition constant or the inhibition in hepatocytes showed that molecular volume, polarisability and molecular surface area are important parameters in determining the rate of inhibition of tolbutamide hydroxylation by sulfonamides in both microsomes and hepatocytes. In addition, log P-oct are also involved in determining inhibition constants in microsomal fractions.DOI:10.1080/004982597240154
文献信息
-
Arenesulfonyl Fluoride Synthesis via Copper-Catalyzed Fluorosulfonylation of Arenediazonium Salts作者:Yongan Liu、Donghai Yu、Yong Guo、Ji-Chang Xiao、Qing-Yun Chen、Chao LiuDOI:10.1021/acs.orglett.0c00484日期:2020.3.20We report herein a general and practical copper-catalyzed fluorosulfonylation reaction of a wide range of abundant arenediazonium salts to smoothly prepare various arenesulfonyl fluorides using the 1,4-diazabicyclo[2.2.2]octane-bis(sulfur dioxide) adduct as a convenient sulfonyl source in combination with KHF2 as an ideal fluorine source and without the need for additional oxidants. Interestingly,
-
[EN] ANTIBACTERIAL COMPOUNDS<br/>[FR] COMPOSÉS ANTIBACTÉRIENS申请人:MASSACHUSETTS GEN HOSPITAL公开号:WO2019199979A1公开(公告)日:2019-10-17The present application provides compounds of formula: Methods of using these compounds for killing bacterial growth and treating bacterial infections are also provided.本申请提供了以下化合物的公式:还提供了使用这些化合物杀灭细菌生长和治疗细菌感染的方法。
-
[EN] CONTROLLED DRUG RELEASE FROM SOLID SUPPORTS<br/>[FR] SUPPORTS SOLIDES POUR LA LIBÉRATION CONTRÔLÉE DE MÉDICAMENTS申请人:PROLYNX LLC公开号:WO2011140392A1公开(公告)日:2011-11-10The invention relates to solid supports useful in medical applications that provide controlled release of drugs, such as peptides, nucleic acids and small molecules. The drugs are covalently coupled to the solid support through a linkage that releases the drug or a prodrug through controlled beta elimination.这项发明涉及在医疗应用中有用的固体支持物,可提供药物的控制释放,如肽、核酸和小分子。这些药物通过一个通过受控β消除释放药物或前药的连接与固体支持物共价偶联。
-
[EN] CONTROLLED RELEASE FROM MACROMOLECULAR CONJUGATES<br/>[FR] LIBÉRATION CONTRÔLÉE À PARTIR DE CONJUGUÉS MACROMOLÉCULAIRES申请人:PROLYNX LLC公开号:WO2011140393A1公开(公告)日:2011-11-10The invention relates to conjugates of macromolecular carriers and drugs comprising linkers that release the drug or a prodrug through rate-controlled beta-elimination, and methods of making and using the conjugates.这项发明涉及大分子载体和药物的共轭物,包括释放药物或前药的连接剂,通过速率控制的β-消除释放药物,以及制备和使用这些共轭物的方法。
-
Novel Thiazole Inhibitors of Fructose 1,6-Bishosphatase申请人:Dang Qun公开号:US20070225259A1公开(公告)日:2007-09-27Compounds of Formula I, their prodrugs and salts, their preparation and their uses are described.公式I的化合物,它们的前药和盐,它们的制备以及它们的用途被描述了。
表征谱图
-
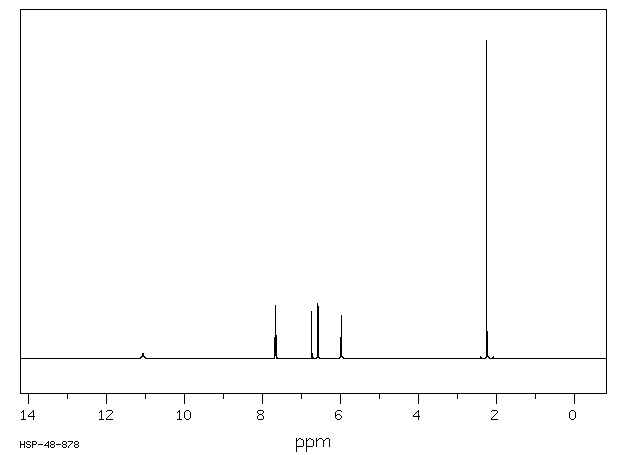
氢谱1HNMR
-
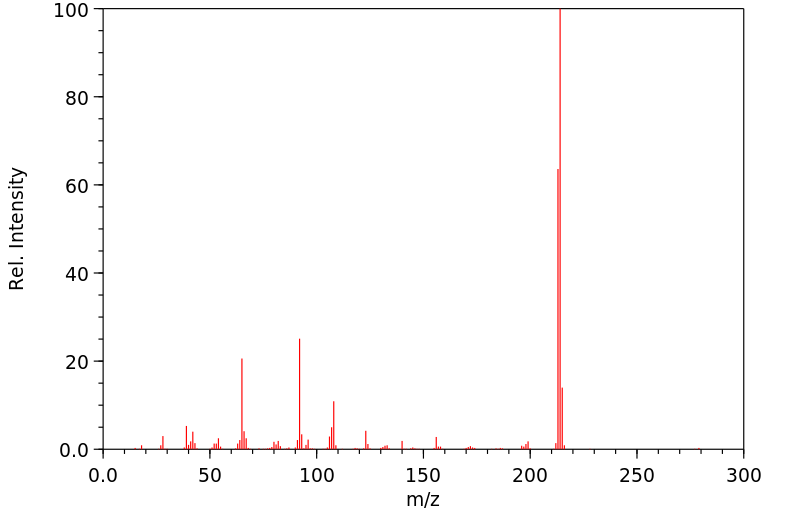
质谱MS
-
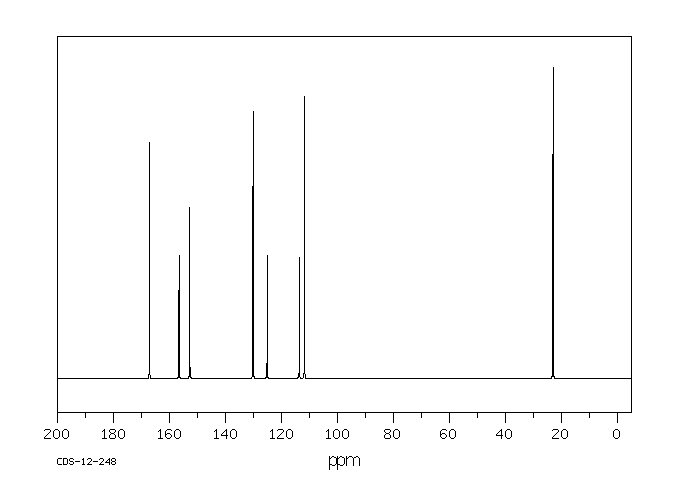
碳谱13CNMR
-
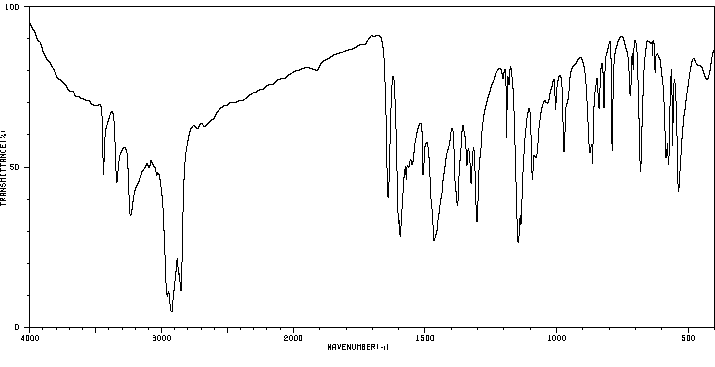
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫