5,6,7,8-四氢-2-喹啉酮 | 54802-19-6
中文名称
5,6,7,8-四氢-2-喹啉酮
中文别名
5,6,7,8-四氢-2(1H)-喹啉酮
英文名称
5,6,7,8-tetrahydro-1H-quinolin-2-one
英文别名
5,6,7,8-tetrahydro-2(1H)-quinolinone;5,6,7,8-tetrahydro-2-quinolinone;5,6,7,8-tetrahydroquinolin-2(1H)-one;5,6,7,8-tetrahydro-2-quinolone
CAS
54802-19-6
化学式
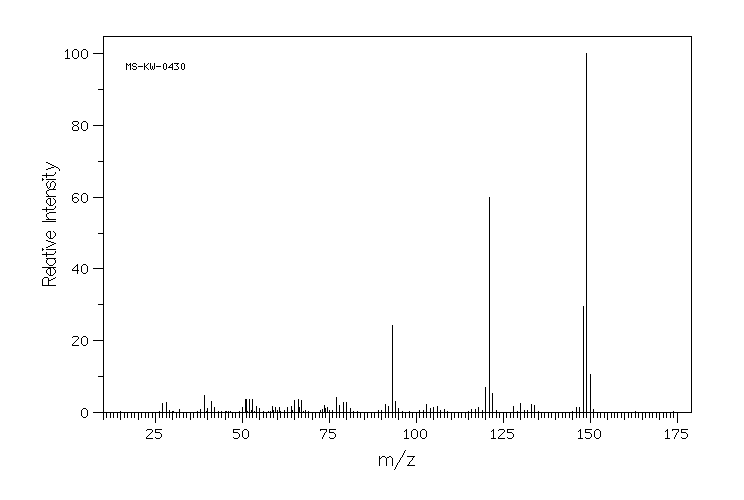
C9H11NO
mdl
MFCD11043839
分子量
149.192
InChiKey
SGKURJURKCHGJG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:204-205℃
-
沸点:402.4±18.0 °C(Predicted)
-
密度:1.13
-
溶解度:DMF:20 mg/ml; DMSO:20 mg/ml;乙醇:30 mg/ml;乙醇P:PBS (pH 7.2)(1:1):0.5 mg/ml
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:11
-
可旋转键数:0
-
环数:2.0
-
sp3杂化的碳原子比例:0.444
-
拓扑面积:29.1
-
氢给体数:1
-
氢受体数:1
安全信息
-
海关编码:2933790090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 5,6,7,8-Tetrahydroquinolin-2(1H)-one
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5,6,7,8-Tetrahydroquinolin-2(1H)-one
CAS number: 54802-19-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H11NO
Molecular weight: 149.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 5,6,7,8-Tetrahydroquinolin-2(1H)-one
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 5,6,7,8-Tetrahydroquinolin-2(1H)-one
CAS number: 54802-19-6
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C9H11NO
Molecular weight: 149.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6,7-二氢-5H环戊[b]并吡啶-2-醇 1,5,6,7-tetrahydro-2H-cyclopentan[b]pyridin-2-one 88499-85-8 C8H9NO 135.166 2-氧代-1,2,5,6,7,8-六氢-3-喹啉甲腈 2-oxo-1,2,5,6,7,8-hexahydroquinoline-3-carbonitrile 4241-13-8 C10H10N2O 174.202 5,6,7,8-四氢-2-甲基喹啉 2-methyl-5,6,7,8-tetrahydroquinoline 2617-98-3 C10H13N 147.22 2-氯-5,6,7,8-四氢喹啉 2-chloro-5,6,7,8-tetrahydro-quinoline 21172-88-3 C9H10ClN 167.638 2-氧代-1,2,5,6,7,8-六氢-喹啉-3-羧酸 2-oxo-1,2,5,6,7,8-hexahydroquinoline-3-carboxylic acid 64500-54-5 C10H11NO3 193.202 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 3-bromo-5,6,7,8-tetrahydro-2(1H)-quinolone 54968-03-5 C9H10BrNO 228.088 2-氯-5,6,7,8-四氢喹啉 2-chloro-5,6,7,8-tetrahydro-quinoline 21172-88-3 C9H10ClN 167.638 2-喹啉甲腈,5,6,7,8-四氢- 5,6,7,8-tetrahydroquinoline-2-carbonitrile 150459-78-2 C10H10N2 158.203 —— 5,6,7,8-tetrahydro-3-nitro-2(1H)-quinolinone 313534-94-0 C9H10N2O3 194.19 2-氯-6,7-二氢喹啉-8(5h)-酮 2-chloro-6,7-dihydro-8(5H)-quinolinone 129337-86-6 C9H8ClNO 181.622 2-氯-8-羟基-5,6,7,8-四氢喹啉 2-chloro-5,6,7,8-tetrahydroquinolin-8-ol 130861-73-3 C9H10ClNO 183.637
反应信息
-
作为反应物:参考文献:名称:4-Benzyl and 4-Benzoyl-3-dimethylaminopyridin-2(1H)-ones: In Vitro Evaluation of New C-3-Amino-Substituted and C-5,6-Alkyl-Substituted Analogues against Clinically Important HIV Mutant Strains摘要:In a program to optimize the anti-HIV activity of the 4-benzyl and 4-benzoyl-3-dimethylaminopyridinones 9 and 10, lead compounds in a new class of highly potent non-nucleoside type inhibitors of HIV-1 reverse transcriptase, modification of the alkyl substitutents at the C-5 and C-6 positions on the pyridinone ring and of the substitutents on the C-3 amino group has been studied. Of the 17 new 5/6-modified analogues prepared, compounds 31b and 32b substituted at C-5 by an extended nonpolar chain containing an ether function and a C-6 methyl group and compound 35 bearing a C-5 ethyl/C-6 hydroxymethyl substituent pattern were selected on the basis of their in vitro activity against wild-type HIV and the three principle mutant strains, K103N, Y181C, and Y188L. When tested further, it was shown that these molecules, and in particular compound 35, are globally more active than 9, 10, and efavirenz against an additional eight single [L100I, K101E, V106A, E138K, V179E, G190A/S, and F227C] and four double HIV mutant strains [L1001 + K103N, K101E + K103N, K103N + Y181C, and F227L + V106A] which are clinically relevant. Concerning modulation of the N-3 substituent, 36 new analogues were prepared. Of these, the N-methyl-N-(2-methoxyethyl)-substituted compounds 40, 42, and 62, as well as the doubly modified compounds 77a and 77b, were selected from the initial screen and were subsequently shown to be active at sub-micromolar concentrations (IC50'S) against all the other mutant strains except K103N + Y181C and F227L + V106A. Two possible, but distinct, modes of binding of these analogues in RT were suggested from molecular modeling studies. The preferred mode of binding for compound 62, corresponding to the predicted "orientation 1", was revealed in the X-ray crystal structure of the compound 62-RT complex.DOI:10.1021/jm0408621
-
作为产物:描述:2-氧代-1,2,5,6,7,8-六氢-3-喹啉甲腈 在 盐酸 作用下, 反应 144.0h, 以70%的产率得到5,6,7,8-四氢-2-喹啉酮参考文献:名称:4-Benzyl and 4-Benzoyl-3-dimethylaminopyridin-2(1H)-ones: In Vitro Evaluation of New C-3-Amino-Substituted and C-5,6-Alkyl-Substituted Analogues against Clinically Important HIV Mutant Strains摘要:In a program to optimize the anti-HIV activity of the 4-benzyl and 4-benzoyl-3-dimethylaminopyridinones 9 and 10, lead compounds in a new class of highly potent non-nucleoside type inhibitors of HIV-1 reverse transcriptase, modification of the alkyl substitutents at the C-5 and C-6 positions on the pyridinone ring and of the substitutents on the C-3 amino group has been studied. Of the 17 new 5/6-modified analogues prepared, compounds 31b and 32b substituted at C-5 by an extended nonpolar chain containing an ether function and a C-6 methyl group and compound 35 bearing a C-5 ethyl/C-6 hydroxymethyl substituent pattern were selected on the basis of their in vitro activity against wild-type HIV and the three principle mutant strains, K103N, Y181C, and Y188L. When tested further, it was shown that these molecules, and in particular compound 35, are globally more active than 9, 10, and efavirenz against an additional eight single [L100I, K101E, V106A, E138K, V179E, G190A/S, and F227C] and four double HIV mutant strains [L1001 + K103N, K101E + K103N, K103N + Y181C, and F227L + V106A] which are clinically relevant. Concerning modulation of the N-3 substituent, 36 new analogues were prepared. Of these, the N-methyl-N-(2-methoxyethyl)-substituted compounds 40, 42, and 62, as well as the doubly modified compounds 77a and 77b, were selected from the initial screen and were subsequently shown to be active at sub-micromolar concentrations (IC50'S) against all the other mutant strains except K103N + Y181C and F227L + V106A. Two possible, but distinct, modes of binding of these analogues in RT were suggested from molecular modeling studies. The preferred mode of binding for compound 62, corresponding to the predicted "orientation 1", was revealed in the X-ray crystal structure of the compound 62-RT complex.DOI:10.1021/jm0408621
文献信息
-
[EN] CDP PROTEIN SECRETION INHIBITORS<br/>[FR] INHIBITEURS DE SÉCRÉTION DE PROTÉINE CDP申请人:KEZAR LIFE SCIENCES公开号:WO2019191527A1公开(公告)日:2019-10-03rovided herein are compounds that inhibit protein secretion, e.g., via inhibition of Sec61. Also provided are compositions of the inhibitor compounds, and methods of using these inhibitors. The compounds disclosed herein can be used, e.g., for the treatment of cancer, arthritis, and/or inflammation.
-
Heterocyclic compounds as inhibitors of factor VIIa申请人:Glunz W. Peter公开号:US20060211720A1公开(公告)日:2006-09-21The present invention relates generally to compounds that inhibit serine proteases. In particular it is directed to novel heterocyclic compounds, or a stereoisomer or pharmaceutically acceptable salt, solvate, or prodrug form thereof, which are useful as selective inhibitors of serine protease enzymes of the coagulation cascade; for example thrombin, factor VIIa, factor Xa, factor XIa, factor IXa, and/or plasma kallikrein. In particular, it relates to compounds that are factor VIIa inhibitors. This invention also relates to pharmaceutical compositions comprising these compounds and methods of using the same.
-
[EN] PYRIDINE DERIVATIVES AND THEIR USE AS MEDICAMENTS FOR TREATING DISEASES RELATED TO MCH RECEPTOR<br/>[FR] DERIVES DE PYRIDINE ET LEUR UTILISATION EN TANT QUE MEDICAMENTS POUR LE TRAITEMENT DES MALADIES LIEES AUX RECEPTEURS MCH申请人:TAISHO PHARMA CO LTD公开号:WO2006035967A1公开(公告)日:2006-04-06The present invention encompasses novel substituted pyridine compounds of Formula (I), which act as MCH receptor antagonists. These compositions and pharmaceutical compositions thereof are useful in the prophylaxis or treatment of improving memory function, sleeping and arousal, anxiety, depression, mood disorders, seizure, obesity, diabetes, appetite and eating disorders, cardiovascular disease, hypertension, dyslipidemia, myocardial infarction, binge eating disorders including bulimia, anorexia, mental disorders including manic depression, schizophrenia, delirium, dementia, stress, cognitive disorders, attention deficit disorder, substance abuse disorders and dyskinesias including Parkinson's disease, epilepsy, and addiction.
-
Pyrid-2-one derivatives and methods of use申请人:——公开号:US20040147561A1公开(公告)日:2004-07-29Selected compounds are effective for treatment of diseases, such as cell proliferation or apoptosis mediated diseases. The invention encompasses novel compounds, analogs, prodrugs and pharmaceutically acceptable derivatives thereof, pharmaceutical compositions and methods for prophylaxis and treatment of diseases and other maladies or conditions involving stroke, cancer and the like. The subject invention also relates to processes for making such compounds as well as to intermediates useful in such processes.所选化合物对于治疗疾病,如细胞增殖或凋亡介导的疾病,具有有效性。该发明涵盖了新颖的化合物、类似物、前药和其药学上可接受的衍生物,以及用于预防和治疗涉及中风、癌症等疾病和其他疾病或病况的药物组合物和方法。该主题发明还涉及制备此类化合物的方法,以及在这些过程中有用的中间体。
-
Novel Potent and Selective Central 5-HT<sub>3</sub> Receptor Ligands Provided with Different Intrinsic Efficacy. 1. Mapping the Central 5-HT<sub>3</sub> Receptor Binding Site by Arylpiperazine Derivatives作者:Andrea Cappelli、Maurizio Anzini、Salvatore Vomero、Laura Mennuni、Francesco Makovec、Edith Doucet、Michel Hamon、Giancarlo Bruni、Maria R. Romeo、M. Cristina Menziani、Pier G. De Benedetti、Thierry LangerDOI:10.1021/jm970645i日期:1998.2.1Synthesis and pharmacological evaluation of a series of condensed quinoline and pyridine derivatives bearing a N-methylpiperazine moiety attached to the 2-position of the quinoline or pyridine nucleus are described. 5-HT receptor binding studies revealed subnanomolar affinity for the 5-HT3 receptor subtype in some of the compounds under study. The most active compound (5b) displayed a Ki value about描述了一系列稠合的喹啉和吡啶衍生物的合成和药理学评价,所述稠合的喹啉和吡啶衍生物带有与喹啉或吡啶核的2-位相连的N-甲基哌嗪部分。5-HT受体结合研究表明,某些化合物对5-HT3受体亚型具有亚纳摩尔亲和力。活性最高的化合物(5b)的Ki值比喹嗪高约1个数量级,而且选择性更高。体外评估了在NG 108-15细胞中依赖5-HT3受体的[14C]胍鎓吸收情况,对四种选定化合物的潜在5-HT3激动剂/拮抗剂活性进行了评估。在此分析中,化合物5j用作5-HT3激动剂,其EC50值接近于喹嗪所报道的值,而5b是部分激动剂,其EC50值约为0.25 nM,化合物5c具有拮抗剂特性,其IC 50值(约8nM)与先前表征的5-HT 3受体拮抗剂的IC 50值在相同范围内。通过使用理论分子描述符进行的定性和定量结构亲和关系研究,可以阐明主要药效学组分的作用,并开发出一种与Quipazine相关的5-HT3配体与其受体相互作用的模型。
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
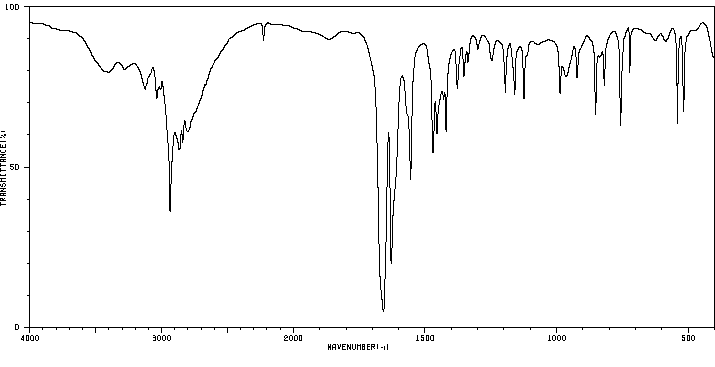
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43