苯乙酰甘氨酸 | 500-98-1
物质功能分类
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:144°C
-
沸点:476.5±38.0 °C(Predicted)
-
密度:1.239±0.06 g/cm3(Predicted)
-
溶解度:少许溶于甲醇
-
物理描述:Solid
-
碰撞截面:147.23 Ų [M-H]- [CCS Type: DT, Method: single field calibrated with Agilent tune mix (Agilent)]
-
稳定性/保质期:
按规格使用和贮存,不会发生分解,避免与氧化物接触。
计算性质
-
辛醇/水分配系数(LogP):0.7
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.2
-
拓扑面积:66.4
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
安全说明:S24/25
-
海关编码:2924299090
-
WGK Germany:3
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H302,H315,H319,H335
-
储存条件:-15°C密封保存,放置在通风、干燥的环境中。
SDS
Section 1. Identification of the substance
Product Name: Phenaceturic acid
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: Phenaceturic acid
CAS number: 500-98-1
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C10H11NO3
Molecular weight: 193.2
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
生物活性化合物2-(2-Phenylacetamido)acetic acid 是一种内源性代谢产物。
| 人类内源性代谢物 |
|---|
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-[(2-苯基乙酰基)氨基]乙酸乙酯 N-(phenylacetyl)glycine ethyl ester 4838-35-1 C12H15NO3 221.256 —— tert-butyl (2-phenylacetyl)glycinate 123910-63-4 C14H19NO3 249.31 —— N-(cyanomethyl)-2-phenylacetamide 5467-51-6 C10H10N2O 174.202 —— phenylthioacetylglycine 872808-70-3 C10H11NO2S 209.269 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N-(2-苯基乙酰基)-甘氨酸甲酯 methyl 2-(2-phenylacetamido)acetate 5259-87-0 C11H13NO3 207.229 —— p-Amino-phenacetyl-glycin 90918-64-2 C10H12N2O3 208.217 2-[(2-苯基乙酰基)氨基]乙酸乙酯 N-(phenylacetyl)glycine ethyl ester 4838-35-1 C12H15NO3 221.256 —— N-phenylacetyl-glycine-anhydride 5874-59-9 C20H20N2O5 368.389 —— benzyl N-phenylacetylglycinate 94359-70-3 C17H17NO3 283.327 —— N-formylphenylacetamide 4252-32-8 C9H9NO2 163.176 —— N-(p-nitrophenylacetyl)-glycine 63257-00-1 C10H10N2O5 238.2 —— N-phenylacetyl-glycine phenyl ester 66591-90-0 C16H15NO3 269.3 —— N-benzyl-2-(2-phenylacetamido)acetamide 15440-34-3 C17H18N2O2 282.342 —— Ethyl 4-((phenylacetyl)amino)-3-oxobutanoate 124022-48-6 C14H17NO4 263.293
反应信息
-
作为反应物:描述:参考文献:名称:Knunjanz; Schokina, Izvestiya Akademii Nauk SSSR, Seriya Khimicheskaya, 1955, p. 462,467; engl. Ausg. S. 409, 413摘要:DOI:
-
作为产物:描述:参考文献:名称:The use of cellulose (chromatography paper) as a cheap, versatile and non-covalent support for organic molecules during multi-step synthesis摘要:纤维素色谱纸提供了一种新颖的非共价支持,用于多维阵列的合成和原位纯化。DOI:10.1039/b208083d
文献信息
-
The Hydrolysis of Esters Related to <i>O</i>-Hippuryl-2-hydroxybutanoic Acid by Carboxypeptidase A作者:John W. Bunting、Joe MurphyDOI:10.1139/v74-385日期:1974.7.15
The hydrolysis of each of the following esters by bovine carboxypeptidase A has been studied at pH 7.5, 25°, ionic strength 0.5: O-hippuryl-, O-phenaceturyl-, O-aceturyl-, O-(N-methylhippuryl)-, and O-(N-hippurylglycyl)-2-hydroxybutanoic acids, and 2-(3-benzoylpropanoxy)-, 2-benzoxyacetoxy-, and 2-(4-phenylbutanoxy)butanoic acids. Substrate inhibition occurs with only the hippuric and phenaceturic acid esters and in the six other cases simple Michaelis–Menten kinetics are observed. The relatively minor variations in the structures of the acid moieties of these esters lead to quite large variations in Km, although kcat seems to be relatively independent of the nature of the acid moiety. Binding modes of substrate molecules at both the catalytic and inhibitory sites are discussed in the light of these observations.
-
[EN] GRANZYME B DIRECTED IMAGING AND THERAPY<br/>[FR] IMAGERIE DU GRANZYME B ET THÉRAPIE DIRIGÉES CONTRE LE GRANZYME B申请人:CYTOSITE BIOPHARMA INC公开号:WO2019160916A1公开(公告)日:2019-08-22Provided herein are heterocyclic compounds useful for imaging Granzyme B. Methods of imaging Granzyme B, combination therapies, and kits comprising the Granzyme B imaging agents are also provided.本文提供了用于成像Granzyme B的杂环化合物。还提供了成像Granzyme B的方法、联合疗法以及包含Granzyme B成像试剂的试剂盒。
-
Low Power Design for ASIC Cores作者:Alvar Dean、David Garrett、Mircea R. Stan、Sebastian VentroneDOI:10.1155/2001/90464日期:2001.1.1
A semicustom ASIC design methodology is used to develop a low power DSP core for mobile (battery powered) applications. Different low power design techniques are used, including dual voltage, low power library elements, accurate power reporting, pseudomicrocode, transition-once logic, clock gating, and others.
使用半定制ASIC设计方法开发低功耗DSP核心,用于移动(电池供电)应用。采用不同的低功耗设计技术,包括双电压、低功耗库元件、准确的功耗报告、伪微码、一次性过渡逻辑、时钟门控等。 -
Ultra-High-Throughput Acoustic Droplet Ejection-Open Port Interface-Mass Spectrometry for Parallel Medicinal Chemistry作者:Kenneth J. DiRico、Wenyi Hua、Chang Liu、Joseph W. Tucker、Anokha S. Ratnayake、Mark E. Flanagan、Matthew D. Troutman、Mark C. Noe、Hui ZhangDOI:10.1021/acsmedchemlett.0c00066日期:2020.6.11(HTE) has emerged as an important tool in drug discovery, providing a platform for preparing large compound libraries and enabling swift reaction screening over wide-ranging conditions. Recent advances in automated high-density, material-sparing HTE have necessitated the development of rapid analytics with sensitivity and resolution sufficient to identify products and/or assess reaction performance in
-
The use of penicillin acylase for selective N-terminal deprotection in peptide synthesis作者:Herbert WaldmannDOI:10.1016/s0040-4039(00)86668-x日期:1988.1Penicillin acylase from E. coli (EC 3.5.1.11) accepts a broad range of N-phenylacetyl-dipeptide esters as substrates. The enzyme hydrolyses the N-terminal protecting group selectively at room temp. and pH=8.1 without affecting the peptide- or the ester-bonds. Alternatively methyl-, benzyl-, tert-butyl and allyl esters can be cleaved chemically leaving the phenylacetamido moiety intact.
表征谱图
-
氢谱1HNMR
-
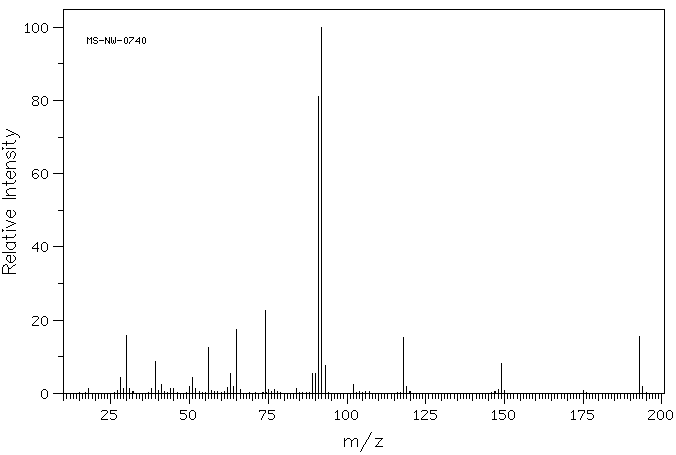
质谱MS
-
碳谱13CNMR
-
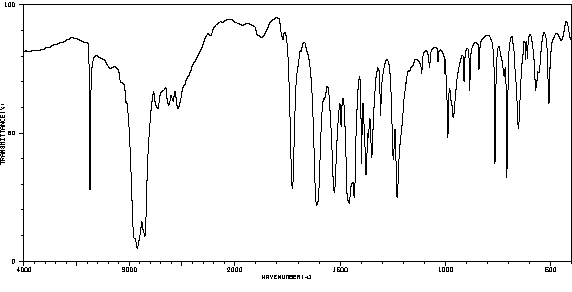
红外IR
-
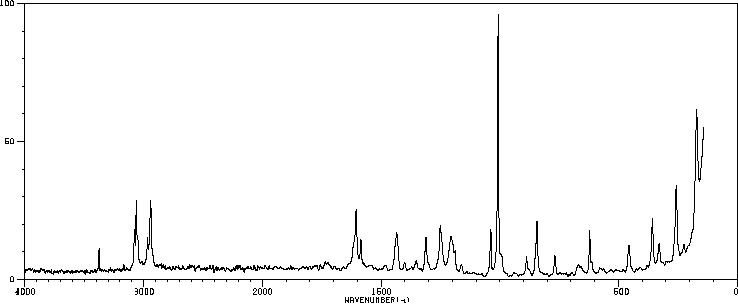
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息