β-L-glucopyranose | 39281-65-7
中文名称
——
中文别名
——
英文名称
β-L-glucopyranose
英文别名
β-D(+)-glucose;L-(-)-glucose;D-Glucose;L-glucose;β-L-glucopyranose;β-L-glucopyranoside;Unii-OG95YI8xcf;(2S,3S,4R,5R,6S)-6-(hydroxymethyl)oxane-2,3,4,5-tetrol
CAS
39281-65-7
化学式
C6H12O6
mdl
——
分子量
180.158
InChiKey
WQZGKKKJIJFFOK-QYESYBIKSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:410.8±45.0 °C(Predicted)
-
密度:1.732±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):-2.6
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:110
-
氢给体数:5
-
氢受体数:6
SDS
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 beta-L-葡萄糖五乙酸酯 β-D-glucose pentaacetate 66966-07-2 C16H22O11 390.344 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (2S,3R,4R,5S,6S)-2-(hydroxymethyl)-6-methoxyoxane-3,4,5-triol —— C7H14O6 194.18 —— 2,3,4,6-tetra-O-acetyl-L-glucoyranose 188913-42-0 C14H20O10 348.307 beta-L-葡萄糖五乙酸酯 β-D-glucose pentaacetate 66966-07-2 C16H22O11 390.344 —— (2R,4aS,6S,7S,8S,8aR)-2-phenyl-4,4a,6,7,8,8a-hexahydropyrano[3,2-d][1,3]dioxine-6,7,8-triol —— C13H16O6 268.266
反应信息
-
作为反应物:描述:β-L-glucopyranose 在 吡啶 、 4-二甲氨基吡啶 、 溶剂黄146 、 乙二胺 作用下, 以 四氢呋喃 为溶剂, 反应 27.0h, 生成 2,3,4,6-tetra-O-acetyl-L-glucoyranose参考文献:名称:Laccase-catalyzed dimerization of glycosylated lignols摘要:Phenylpropanoid glucosides (PPGs) are naturally occurring and bioactive phenolic derivatives, largely distributed in plants. In this work different PPGs have been chemically or enzymatically synthesized from the lignols coniferyl and p-coumaryl alcohols as substrates for a lactase-catalyzed oxidative coupling. The biooxidation of these PPGs has been investigated here and novel dihydrobenzofuran-based structurally modified analogues have been isolated and characterized. Specifically, the presence of a carbohydrate moiety increased the water solubility of these compounds and reduced the number of dimeric products, as pinoresinol-like structures could not be formed. Looking for a possible sugar-promoted stereochemical enrichment of the obtained diastereomeric mixtures of dimers, different carbohydrate moieties (D-glucose, L-glucose and the disaccharide rutinose) were considered and the respective d.e. values of the dimeric products were measured by H-1 NMR and HPLC. However, it was found that the sugar substituent had a minor effect on the stereochemical outcome of the radical coupling reactions, the best measured result being a d.e. value of 21%. (C) 2016 Elsevier B.V. All rights reserved.DOI:10.1016/j.molcatb.2016.10.019
-
作为产物:描述:参考文献:名称:来自Onychium japonicum的抗炎新型氰基二萜糖苷摘要:Onychium japonicum (Thunb.) O. Kuntze (Pteridaceae) 地上部分的化学探索提供了两种新的氰基二萜糖苷(1和2)。基于 HRESIMS 和 1D 和 2D NMR 光谱数据分析明确地进行了新化合物的结构解析。这两种化合物代表具有吡喃葡萄糖基部分的前两种氰基二萜。化合物1和2对 RAW246.7 巨噬细胞中 LPS 诱导的一氧化氮 (NO) 产生表现出有效的抑制作用,其 IC 50值分别为 1.76 和 2.82 μM。DOI:10.1016/j.phytol.2021.06.002
-
作为试剂:描述:1-deoxy-1-nitro-L-glucitol 、 sodium hydroxide 、 硫酸 、 、 氢氧化钡 在 硫酸酯 、 Barium acetate, powder 、 barium sulfate 、 乙醇 、 β-L-glucopyranose 作用下, 以 水 为溶剂, 生成 L-(-)-葡萄糖参考文献:名称:Cherry tree named ‘Prim 25’摘要:一种新的、独特的甜樱桃树品种,产生深红色的水果,果肉非常坚实。公开号:USPP033719P2
文献信息
-
GLYCOSIDE COMPOUND AND PREPARATION METHOD THEREFOR, COMPOSITION, APPLICATION, AND INTERMEDIATE申请人:SHANGHAI HUTCHISON PHARMACEUTICALS LIMITED公开号:US20210115082A1公开(公告)日:2021-04-22The present invention discloses a glycoside compound represented by Formula III, and a preparation method, a composition, use and an intermediate thereof. The glycoside compound provided in the present invention has simple preparation method, can significantly increase the expression of VEGF-A mRNA, and is effective in promoting the angiogenesis. This provides a reliable guarantee for the development of drugs with pro-angiogenic activity for treating cerebral infarction cerebral stroke, myocardial infarction, and ischemic microcirculatory disturbance of lower limbs.本发明公开了一种由III式表示的糖苷化合物,以及其制备方法、组合物、用途和中间体。本发明提供的糖苷化合物具有简单的制备方法,可以显著增加VEGF-A mRNA的表达,并且在促进血管生成方面具有有效性。这为开发具有促血管生成活性的药物治疗脑梗死、脑卒中、心肌梗死和下肢缺血微循环障碍提供了可靠的保证。
-
Determination of the absolute configuration of monosaccharides by 1H NMR spectroscopy of their per-O-(S)-2-methylbutyrate derivatives作者:William S. York、Stephen Hantus、Peter Albersheim、Alan G. DarvillDOI:10.1016/s0008-6215(97)00050-5日期:1997.5Abstract An empirical method was developed to determine the absolute configuration of monosaccharides, based on high-field 1H NMR spectroscopy of their per- O-(S)-2-methylbutyrate (SMB) derivatives. The SMB derivatives of the D and L forms of a given monosaccharide are diastereomers, allowing them to be distinguished on the basis of differences in their 1H NMR chemical shifts. The reproducibility of
-
Nouveaux flavonosides dePaeonia tenuifolia L作者:Du??ica Sto?i?、Mom?ilo Gorunovi?、Alexios-L�andros Skaltsounis、Fran�ois Tillequin、Miechel KochDOI:10.1002/hlca.19880710207日期:1988.3.16New Flavonoid Glycosides from Paeonia tenuifolia L.新黄酮苷的芍药细叶L.
-
Pharmaceutically active compounds and their use申请人:Laboratorio CHile S.A.公开号:US05747462A1公开(公告)日:1998-05-05The present invention relates to the area of pharmacology; its objective is to solve the technical problem of inflammation, pain, pruritus and local hyperthermia in human beings and animal species. The composition and the subcompositions thereof are obtained from plants of the family Cactaceae, the main methodological steps being a set of processes: production, purification, physicochemical quantification, biotherapeutic evaluation, biopharmaceutical formulation and molecular identification. From the molecular identification a set of molecules is recognized, comprising carbohydrates and an aromatic amine, the general formulae of which are: C.sub.5 H.sub.10 O.sub.5 (RIBOSE), C.sub.6 H.sub.12 O.sub.5 (FUCOSE), C.sub.6 H.sub.12 O.sub.6 (GALACTOSE; MANNOSE; GLUCOSE), C.sub.8 H.sub.11 O.sub.2 N (1-HYDROXY-1-(4-HYDROXYPHENYL)-2-AMINOETHANE), C.sub.10 H.sub.18 O.sub.9 (RIBOFURANOSYLRIBOSE).本发明涉及药理学领域,其目的是解决人类和动物物种中的炎症、疼痛、瘙痒和局部高热问题。该组合物及其亚组分是从仙人掌科植物中获得的,其主要方法步骤包括一系列过程:生产、纯化、物理化学定量、生物治疗评估、生物制药配方和分子鉴定。从分子鉴定中识别出一组分子,包括碳水化合物和芳香胺,其通用式为:C.sub.5 H.sub.10 O.sub.5(核糖)、C.sub.6 H.sub.12 O.sub.5(岩藻糖)、C.sub.6 H.sub.12 O.sub.6(半乳糖;甘露糖;葡萄糖)、C.sub.8 H.sub.11 O.sub.2 N(1-羟基-1-(4-羟基苯基)-2-氨基乙烷)、C.sub.10 H.sub.18 O.sub.9(核糖呋喃糖核糖)。
-
DEVICE FOR AUTOMATED SYNTHESIS OF OLIGO- AND POLYSACCHARIDES申请人:Max-Planck-Gesellschat zu rFérderung der Wissenschaften e.V.公开号:US20220395800A1公开(公告)日:2022-12-15The present invention generally relates to automated synthesis technology, and more particularly, to a device and method for automated synthesis of oligo- and polysaccharides on a solid support. In particular the present invention relates to a device for automated synthesis of oligo- and polysaccharides on a solid support comprising a reaction vessel, a reagent storing component, a reagent delivery system, a cooling device for cooling reaction vessel, and a pre-cooling device for pre-cooling the reagents to be supplied.
表征谱图
-
氢谱1HNMR
-
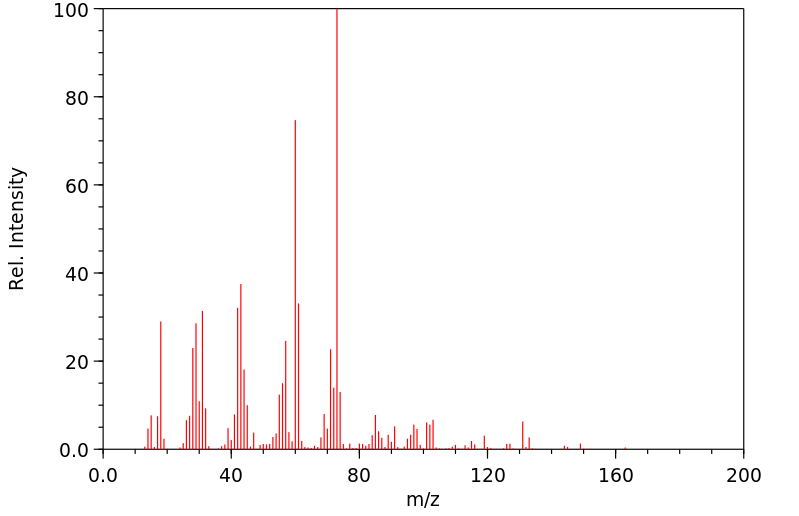
质谱MS
-
碳谱13CNMR
-
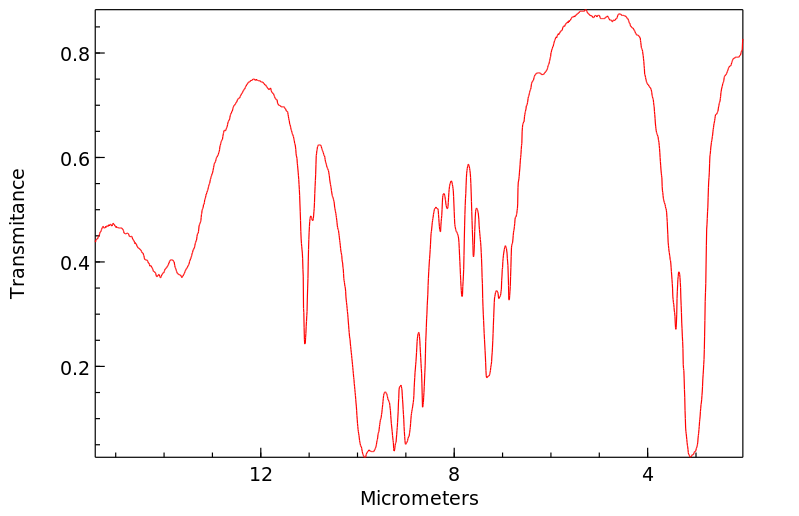
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷