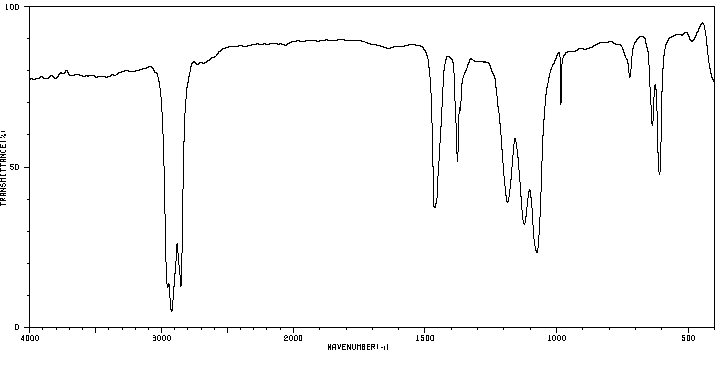
代谢
Barium sulfate is poorly water soluble and shows negligible levels of absorption from the gastrointestinal tract following both oral or rectal administration. In healthy subjects, orally administered barium sulfate is generally excreted within 24 hours. Rectally administered barium sulfate is eliminated with clearance of the enema.
来源:DrugBank