4-氯-4'-硝基二苯甲酮 | 13736-61-3
分子结构分类
中文名称
4-氯-4'-硝基二苯甲酮
中文别名
——
英文名称
(2E)-1-(4-chlorophenyl)-3-(4-nitrophenyl)prop-2-en-1-one
英文别名
(E)-1-(4-chlorophenyl)-3-(4-nitrophenyl)prop-2-en-1-one
CAS
13736-61-3
化学式
C15H10ClNO3
mdl
——
分子量
287.702
InChiKey
JBXAGWCLKWPVFB-XCVCLJGOSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):3.5
-
重原子数:20
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:62.9
-
氢给体数:0
-
氢受体数:3
SDS
上下游信息
反应信息
-
作为反应物:参考文献:名称:Annapoorna; Prasad Rao; Sethuram, Indian Journal of Chemistry, Section A: Inorganic, Physical, Theoretical and Analytical, 2001, vol. 40, # 3, p. 283 - 287摘要:DOI:
-
作为产物:描述:对硝基苯甲醛 以98%的产率得到参考文献:名称:TAO, WENTIAN;HU, XIANMING, CHEM. REAGENTS, 1984, 6, N 4, 207-209摘要:DOI:
文献信息
-
SAR and molecular mechanism studies of monoamine oxidase inhibition by selected chalcone analogs作者:Raed Shalaby、Jacobus P. Petzer、Anél Petzer、Usman M. Ashraf、Ealla Atari、Fawaz Alasmari、Sivarajan Kumarasamy、Youssef Sari、Ashraf KhalilDOI:10.1080/14756366.2019.1593158日期:2019.1.1reversible competitive mode of inhibition. Most of the synthesized chalcone analogs showed a better selectivity toward MAO-B. However, introducing of 2,4,6-trimethoxy substituents on ring B shifted the selectivity toward MAO-A. In addition, we investigated the molecular mechanism of MAO-B inhibition by selected chalcone analogs. Our results revealed that these selected chalcone analogs increased dopamine levels抽象的 本研究描述了一系列22查尔酮类似物的合成。这些化合物被评估为潜在的人类MAO-A和MAO-B抑制剂。化合物对两种同工型表现出不同的选择性。发现IC 50值在微摩尔至亚微摩尔范围内。该ķ我化合物16的MAO-A和MAO-B抑制值分别为0.047和0.020μM。酶抑制剂混合物的透析表明抑制作用是可逆的竞争方式。大多数合成的查耳酮类似物对MAO-B表现出更好的选择性。然而,在环B上引入2,4,6-三甲氧基取代基使选择性向MAO-A转移。此外,我们研究了所选查耳酮类似物抑制MAO-B的分子机制。我们的结果表明,这些选定的查尔酮类似物可增加大鼠肝癌(H4IIE)细胞中的多巴胺水平,并降低MAO-B酶的相对mRNA表达。
-
Effects of Structural and Electronic Characteristics of Chalcones on the Activation of Peroxisome Proliferator-Activated Receptor Gamma作者:Jason Taylor Schott、Charles Edward Mordaunt、Anthony Joseph Vargas、Martin Antonio Leon、Kevin Hsinwen Chen、Mandeep Singh、Mikiko Satoh、Emilio Leal Cardenas、Santanu Maitra、Nilay Vinod Patel、Hubrecht Johan Peter de LijserDOI:10.1248/cpb.c12-00749日期:——chalcones with an electron rich group or sterically large groups such as naphthyl on the carbonyl side tend to activate PPARγ. The absence of any strict structural or electronic requirements suggests that the flexibility of the PPARγ ligand binding pocket may allow binding of diverse chalcones with some preference for a slightly larger electron-rich group on the carbonyl side. We predict that further structure-activity
-
Electronic effects in the regioselectivity of nucleophilic attacks on cationic 1,3-diaryl-π-allylpalladium complexes作者:Maria Prat、Jordi Ribas、Marcial Moreno-MañasDOI:10.1016/s0040-4020(01)88728-7日期:1992.2Nucleophilic attacks on π-allylpalladium complexes derived from O2NPh(CHCHCH)PhX systems (X = 4-OMe and 4-Cl) occur prefentially at the allylic terminus remote from the electron-withdrawing group (NO2).上π烯丙基钯络合物的亲核攻击选自O衍生2 NPh(CHCHCH)PhX系统(X = 4-OME和4- Cl)的从烯丙基末端prefentially发生远程吸电子组(NO 2)。
-
One-Pot Synthesis of α,β-Unsaturated Ketones作者:Kilsung Lee、Dong Young OhDOI:10.1055/s-1991-26423日期:——The preparation of α,β-unsaturated ketones is performed in a one-pot procedure by subsequent treatment of diethyl lithiomethyl-phosphonate with nitriles, followed by addition of carbonyl compounds and hydrolysis.
-
Novel Potent and Selective DPP-4 Inhibitors: Design, Synthesis and Molecular Docking Study of Dihydropyrimidine Phthalimide Hybrids作者:Ahmed A. E. Mourad、Ahmed E. Khodir、Sameh Saber、Mai A. E. MouradDOI:10.3390/ph14020144日期:——Methods: A novel series of dihydropyrimidine phthalimide hybrids was synthesized and evaluated for their in vitro and in vivo DPP-4 inhibition activity and selectivity using alogliptin as reference. Oral glucose tolerance test was assessed in type 2 diabetic rats after chronic treatment with the synthesized hybrids ± metformin. Cytotoxicity and antioxidant assays were performed. Additionally, molecular docking背景:二肽基肽酶 4 (DPP-4) 抑制剂已作为抗高血糖药物出现,可改善 2 型糖尿病患者的血糖控制,无论是作为单一疗法还是与其他抗糖尿病药物联合使用。方法:合成了一系列新型二氢嘧啶邻苯二甲酰亚胺杂化物,并以阿格列汀为参考,评估了其体外和体内 DPP-4 抑制活性和选择性。在用合成的混合物±二甲双胍长期治疗后,对2型糖尿病大鼠进行了口服葡萄糖耐量试验的评估。进行了细胞毒性和抗氧化剂测定。此外,还对新型杂交体与DPP-4的分子对接研究和构效关系进行了研究。结果:合成的杂交体中, 10g、 10i 、 10e 、 10d 、 10b的体外DPP-4抑制活性强于阿格列汀。此外,体内 DPP-4 抑制测定显示,与阿格列汀相比, 10g和10i具有最强和最持久的血液 DPP-4 抑制活性。在 2 型糖尿病大鼠中,混合物10g 、 10i和10e表现出比阿格列汀更好的血糖控制,二甲双胍组合进一步支持了这一效果。最后,在DPPH测定中,
表征谱图
-
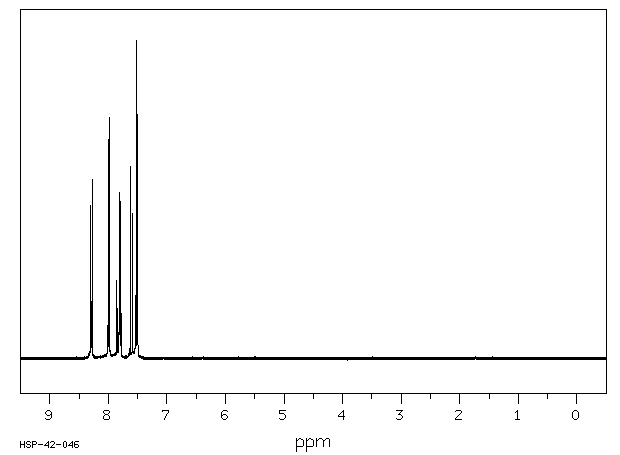
氢谱1HNMR
-
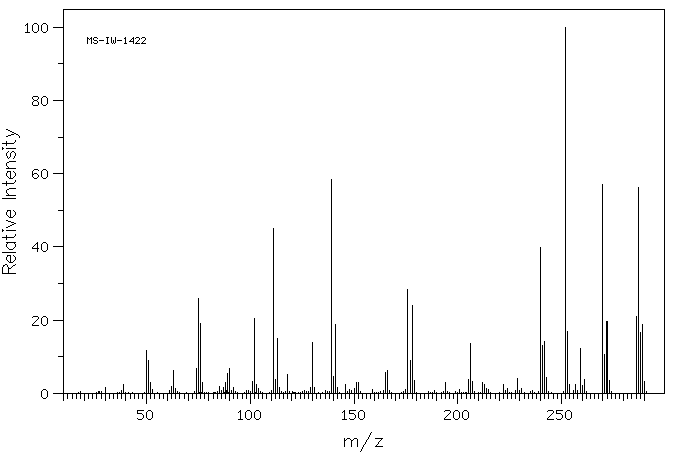
质谱MS
-
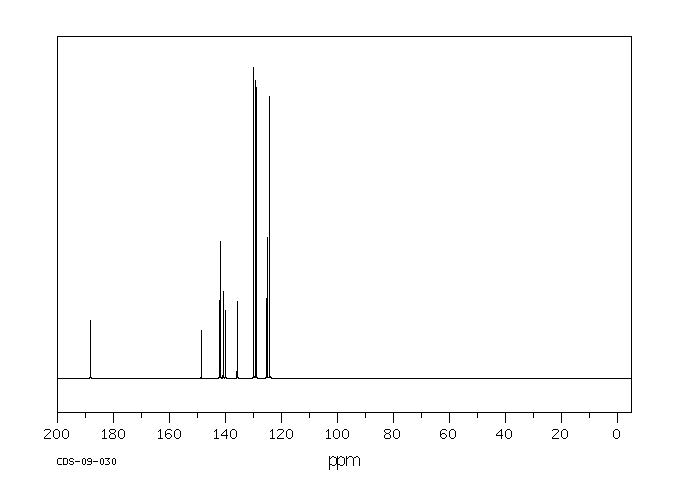
碳谱13CNMR
-
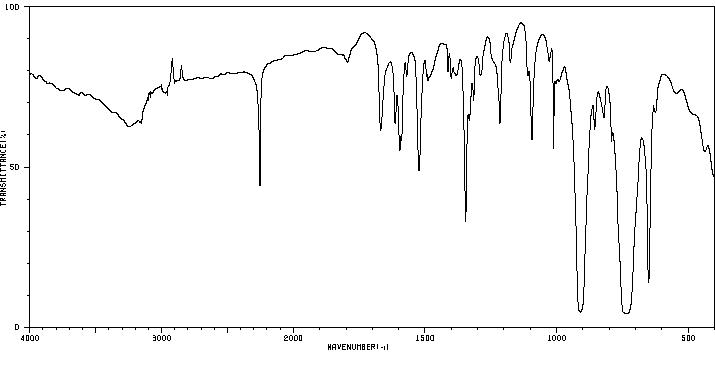
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2Z)-1,3-二苯基-2-丙烯-1-酮,2-丙烯-1-酮,1,3-二苯基-,(2Z)-
龙血素D
龙血素A
龙血素 B
黄色当归醇F
黄色当归醇B
黄腐醇; 黄腐酚
黄腐醇 D; 黄腐酚 D
黄腐酚B
黄腐酚
黄腐酚
黄卡瓦胡椒素 C
高紫柳查尔酮
阿普非农
阿司巴汀
阿伏苯宗
金鸡菊查耳酮
邻肉桂酰苯甲酸
达泊西汀杂质25
豆蔻明
补骨脂色烯查耳酮
补骨脂查耳酮
补骨脂呋喃查耳酮
补骨脂乙素
蜡菊亭; 4,2',4'-三羟基-6'-甲氧基查耳酮
苯酚,4-[3-(2-羟基苯基)-1-苯基丙基]-2-(3-苯基丙基)-
苯磺酰胺,N-[4-[3-(3-羟基苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,N-[3-[3-(4-羟基-3-甲氧苯基)-1-羰基-2-丙烯基]苯基]-
苯磺酰胺,4-甲氧基-N,N-二甲基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯化,4,5-二甲氧基-2-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯磺酰氯,4-甲氧基-3-(3-羰基-3-苯基-1-丙烯基)-,(E)-
苯甲醇,4-甲氧基-a-[2-(4-甲氧苯基)乙烯基]-
苯甲酸-[4-(3-氧代-3-苯基-丙烯基)-苯胺]
苯甲酸,3-[3-(4-溴苯基)-1-羰基-2-丙烯基]-4-羟基-
苯甲酰(2-羟基苯酰)甲烷
苯甲腈,4-(1-羟基-3-羰基-3-苯基丙基)-
苯基[2-(1-萘基)乙烯基]甲酮
苯基-(三苯基-丙-2-炔基)-醚
苯基-(2-苯基-2,3-二氢-苯并噻唑-2-基)-甲酮
苯亚甲基苯乙酮
苯乙酰腈,a-(1-氨基-2-苯基亚乙基)-
苯丙酸,a-苯甲酰-b-羰基-,苯基(苯基亚甲基)酰肼
苯,1-(2,2-二甲基-3-苯基丙基)-2-甲基-
苏木查耳酮
苄桂哌酯
苄基(4-氯-2-(3-氧代-1,3-二苯基丙基)苯基)氨基甲酸酯
芦荟提取物
腈苯唑
胀果甘草宁C
聚磷酸根皮酚