phenanthridin-6-yl(phenyl)methanone | 91871-07-7
中文名称
——
中文别名
——
英文名称
phenanthridin-6-yl(phenyl)methanone
英文别名
6-benzoylphenanthridine;9-Benzoyl-phenanthridin
CAS
91871-07-7
化学式
C20H13NO
mdl
——
分子量
283.329
InChiKey
XYBIHNLSMJZMFS-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
计算性质
-
辛醇/水分配系数(LogP):5.1
-
重原子数:22
-
可旋转键数:2
-
环数:4.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:30
-
氢给体数:0
-
氢受体数:2
反应信息
-
作为反应物:描述:phenanthridin-6-yl(phenyl)methanone 在 N-苯基哌啶 、 DPZ 、 C31H21F6O4P 作用下, 反应 72.5h, 以99%的产率得到(R)-phenanthridin-6-yl(phenyl)methanol参考文献:名称:通过可见光驱动的光氧化还原不对称催化 对氮杂芳烃基酮的对映选择性还原†摘要:据报道,通过光氧化还原不对称催化,对氮杂芳烃基酮的对映选择性还原。借助包含手性磷酸和光敏剂DPZ的无过渡金属双催化体系,该体系由可见光介导,转化过程涉及串联过程,该过程涉及双单电子转移还原和对映选择性质子化,从而以高收率提供了有价值的手性醇(向上至> 99%)具有良好至优异的对映选择性(高达97%ee)。DOI:10.1039/c9cc03661j
-
作为产物:描述:2-氨基联苯 在 sodium persulfate 、 乙酸酐 、 silver carbonate 作用下, 以 四氢呋喃 、 二甲基亚砜 为溶剂, 反应 10.0h, 生成 phenanthridin-6-yl(phenyl)methanone参考文献:名称:Synthesis of 6-acyl phenanthridines by oxidative radical decarboxylation–cyclization of α-oxocarboxylates and isocyanides摘要:一种通过氧化性自由基脱羧-环化反应合成6-酰基菲啰啉的银催化方法已经开发出来。DOI:10.1039/c3cc49026b
文献信息
-
Photoredox-Catalyzed Decarboxylative C–H Acylation of Heteroarenes作者:Chao Yang、Wujiong Xia、Wei Jia、Yong Jian、Binbin HuangDOI:10.1055/s-0037-1609911日期:2018.9A mild, environmentally friendly, and regioselective acylation of heterocycles with inexpensive carboxylic acids is reported via photoredox catalysis. The strategy is highlighted with good functional group tolerance and substrate scope which could rapidly realize the acylation of various heterocyclic compounds.
-
Visible-Light-Initiated, Photocatalyst-Free Decarboxylative Coupling of Carboxylic Acids with <i>N</i>-Heterocycles作者:Xiao-Yu Zhang、Wei-Zhi Weng、Hao Liang、Hua Yang、Bo ZhangDOI:10.1021/acs.orglett.8b02016日期:2018.8.3protocol for direct C–H alkylation and acylation of N-heterocycles, using readily accessible carboxylic acids as radical precursors under visible-light irradiation without a photocatalyst and an additional acid additive, has been developed. This protocol provides expedient access to substituted N-heterocycles under mild and metal-free conditions. Mechanistic experiments indicate that this reaction proceeds
-
Synthesis of 6-aroyl phenanthridines by Fe-catalyzed oxidative radical cyclization of 2-isocyanobiphenyls with benzylic alcohols作者:Ziyi Nie、Qiuping Ding、Yiyuan PengDOI:10.1016/j.tet.2016.11.010日期:2016.12A practical method for the synthesis of 6-aroyl phenanthridine derivatives by Fe-catalyzed oxidative radical cyclization of 2-isocyanobiphenyls with benzylic alcohols is described. In addition, this cyclization could be occurred by using toluene as aroyl source. The procedure tolerates various functional groups under simple conditions. A single-electron-transfer pathway is proposed according to mechanistic
-
Visible-Light-Mediated Direct Decarboxylative Acylation of Electron-Deficient Heteroarenes Using α-Ketoacids作者:Sabyasachi Manna、Kandikere Ramaiah PrabhuDOI:10.1021/acs.joc.9b00004日期:2019.5.3Acylation of electron-deficient heteroaromatic compounds has been developed using visible light. α-Ketoacids have been used as an efficient source of acyl radicals under photoredox conditions. The in situ generated acyl radicals from α-ketoacids have been coupled to a wide variety of electron-deficient heteroaromatic compounds in a Minisci type reaction. This method would be attractive to access biologically
-
Selective C(sp<sup>3</sup>)–H activation of simple alkanes: visible light-induced metal-free synthesis of phenanthridines with H<sub>2</sub>O<sub>2</sub> as a sustainable oxidant作者:Yongqiang Zhang、Yunhe Jin、Lifang Wang、Qingqing Zhang、Changgong Meng、Chunying DuanDOI:10.1039/d1gc02670d日期:——
A visible light-induced metal-free C(sp3)–H phenanthridinylation of simple alkanes with isonitrile is developed with H2O2 as a terminal sustainable oxidant.
表征谱图
-
氢谱1HNMR
-
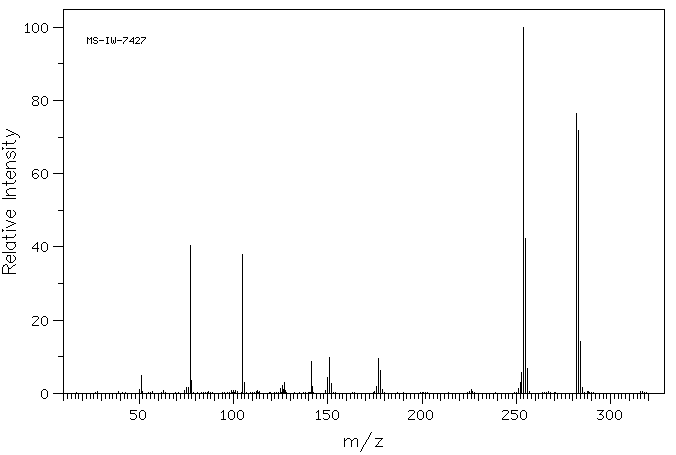
质谱MS
-
碳谱13CNMR
-
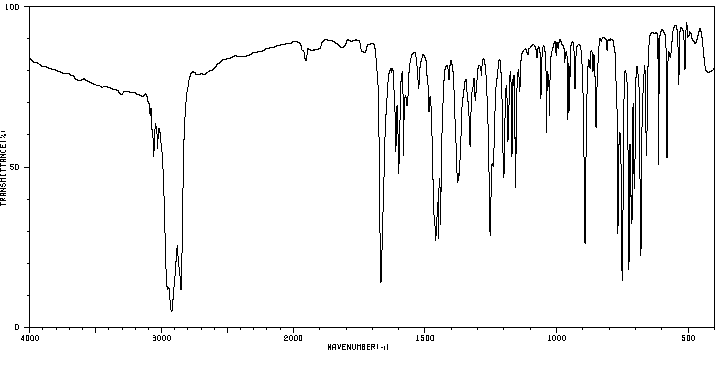
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43