3-哌啶基-1,2-丙二醇 | 4847-93-2
中文名称
3-哌啶基-1,2-丙二醇
中文别名
——
英文名称
3-piperidin-1-yl-propane-1,2-diol
英文别名
3-piperidino-propane-1,2-diol;(+/-)-3-Piperidino-propan-1,2-diol;3-Piperidino-propan-1,2-diol;3-(piperidin-1-yl)propane-1,2-diol;3-piperidino-1,2-propanediol;3-piperidin-1-ylpropane-1,2-diol
CAS
4847-93-2
化学式
C8H17NO2
mdl
MFCD00006506
分子量
159.228
InChiKey
MECNWXGGNCJFQJ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:77-80 °C (lit.)
-
沸点:284.73°C (rough estimate)
-
密度:1.0469 (rough estimate)
计算性质
-
辛醇/水分配系数(LogP):-0.2
-
重原子数:11
-
可旋转键数:3
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:43.7
-
氢给体数:2
-
氢受体数:3
安全信息
-
危险品标志:T
-
危险类别码:R25,R41,R37/38
-
危险品运输编号:UN 2811 6.1/PG 3
-
安全说明:S24/25
-
WGK Germany:3
-
危险标志:GHS05,GHS06
-
危险性描述:H300,H315,H318,H335
-
危险性防范说明:P261,P264,P280,P301 + P310,P305 + P351 + P338
-
储存条件:室温
SDS
| Name: | 3-Piperidino-1 2-propanediol 96% Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 4847-93-2 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4847-93-2 | 3-piperidino-1,2-propanediol | 96.0 | 225-438-1 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Antidote: None reported.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 4847-93-2: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Crystalline powder
Color: light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 77.00 - 80.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: N/A
Explosion Limits, upper: N/A
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C8H17NO2
Molecular Weight: 159.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4847-93-2 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
3-piperidino-1,2-propanediol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Other No information available.
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 4847-93-2: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4847-93-2 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4847-93-2 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1-哌啶基乙酸 piperidinoacetic acid 3235-67-4 C7H13NO2 143.186
反应信息
-
作为反应物:描述:3-哌啶基-1,2-丙二醇 在 氧气 、 sodium methylate 、 silver trifluoromethanesulfonate 作用下, 以 四氢呋喃 、 甲醇 为溶剂, 37.0 ℃ 、101.33 kPa 条件下, 以65%的产率得到1-哌啶基乙酸参考文献:名称:银(I)催化广泛适用的有氧1,2-二元氧化裂解摘要:1,2-二醇的氧化裂解是一种基本的有机转化。仍然主要用于这种氧化裂解的化学计量氧化剂,例如H 5 IO 6 ,Pb(OAc)4 和KMnO 4 ,会产生化学计量危险废物。在本文中,我们描述了一种广泛应用的,高度选择性的银(I)催化的1,2-二醇的氧化裂解,该裂解消耗大气中的氧气作为唯一氧化剂,因此可作为经典转化的潜在绿色替代方法。DOI:10.1002/anie.201711531
-
作为产物:描述:1-[(2,2-dimethyl-1,3-dioxolan-4-yl)methyl]piperidine 在 盐酸 、 水 、 sodium methylate 作用下, 以 甲醇 为溶剂, 以77%的产率得到3-哌啶基-1,2-丙二醇参考文献:名称:Water-soluble meroterpenes containing an aminoglyceride fragment with geraniol residues: synthesis and membranotropic properties摘要:A number of new membrane anchors based on water-soluble aminoglycerides with geraniol fragments have been synthesized. A biomimetic approach was used based on the design of meroterpenes structurally similar to archaeal lipids. Turbidimetry and laser Doppler microelectrophoresis showed that the synthesized compounds were incorporated into unilamellar dipalmitoylphosphatidylcholine (DPPC) vesicles.DOI:10.1016/j.mencom.2019.01.008
-
作为试剂:参考文献:名称:Process for the preparation of alkylene glycols摘要:本发明揭示了一种从相应的环氧烷制备烷基二醇的方法,例如从环氧乙烷制备乙二醇,在水、催化剂和可选的二氧化碳的存在下进行。催化剂含有一种两性化合物,例如乙二胺四乙酸(EDTA)。这些双官能化合物具有酸和碱官能团。优选地,本发明中有用的化合物在水中形成缓冲溶液,即酸和碱官能团不完全解离。缓冲溶液的pH值应为2-10,优选为5-10,更优选为4-9。本发明中有用的化合物优选为有机化合物,其中碱官能团和酸官能团之间相隔一个至四个碳原子。公开号:US20060122437A1
文献信息
-
JAK INHIBITOR申请人:Kyowa Hakko Kirin Co., Ltd.公开号:EP2108642A1公开(公告)日:2009-10-14A JAK inhibitor comprising, as an active ingredient, a nitrogen-containing heterocyclic compound represented by formula (I) wherein W represents a nitrogen atom or -CH-; X represents -C (=O) - or -CHR4- (wherein R4 represents a hydrogen atom, or the like); R1 represents the formula described below [wherein Q1 represents-CR8-(wherein R8 represents a hydrogen atom, substituted or unsubstituted lower alkyl, or the like); Q2 represents -NR15- (wherein R15 represents a hydrogen atom, substituted or unsubstituted lower alkyl, or the like); and R5 and R6 may be the same or different and each represents a hydrogen atom, halogen, carboxy, substituted or unsubstituted lower alkyl, or the like], or the like; and R2 and R3 may be the same or different and each represents a hydrogen atom, halogen, substituted or unsubstituted lower alkyl, or the like} or a pharmaceutically acceptable salt thereof.
-
[EN] QUINAZOLINE DERIVATIVES FOR USE IN THE TREATMENT OF CANCER<br/>[FR] DERIVES DE QUINAZOLINE UTILISES DANS LE TRAITEMENT DU CANCER申请人:ASTRAZENECA AB公开号:WO2004004732A1公开(公告)日:2004-01-15The invention concerns quinazoline derivatives of Formula (I) wherein each of Z, m, R1, n, R3,Z2 and R14 have any of the meanings defined hereinbefore in the description; processes for their preparation, pharmaceutical compositions containing them and their use in the manufacture of a medicament for use as an anti-invasive or anti-proliferative agent in the containment and/or treatment of solid tumour disease.
-
MANUFACTURING METHOD OF HYDROFLUOROOLEFIN申请人:ASAHI GLASS COMPANY, LIMITED公开号:US20180037524A1公开(公告)日:2018-02-08A method for manufacturing hydrofluoroolefin, includes: converting hydrofluorocarbon represented by a formula (1) into hydrofluoroolefin (HFO) represented by formula (2) in the presence of carbon dioxide to obtain a first gas composition containing hydrofluoroolefin and carbon dioxide; and separating carbon dioxide contained in the first gas composition to obtain a second gas composition containing HFO, CR 1 R 2 X 1 CR 3 R 4 X 2 . . . (1), CR 1 R 2 ═CR 3 R 4 . . . (2), wherein R 1 to R 3 are each independently hydrogen atom or fluorine atom, R 4 is hydrogen atom, fluorine atom, CH 3 , CH 2 F, CHF 2 or CF 3 , the total number of fluorine atoms of R 1 to R 4 is one or more, and the total number of hydrogen atoms of R 1 to R 4 is one or more, X 1 and X 2 are each hydrogen atom or fluorine atom where X 2 is the fluorine atom when X 1 is the hydrogen atom, and X 2 is the hydrogen atom when X 1 is the fluorine atom.
-
Novel sulfonyldiazomethanes, photoacid generators, resist compositions, and patterning process申请人:——公开号:US20040167322A1公开(公告)日:2004-08-26A chemical amplification type resist composition comprising a specific benzenesulfonyldiazomethane containing a long-chain alkoxyl group at the 2-position on benzene ring has many advantages including improved resolution, improved focus latitude, minimized line width variation or shape degradation even on long-term PED, minimized debris left after coating, development and peeling, and improved pattern profile after development and is thus suited for microfabrication.
-
[EN] QUINAZOLINE DERIVATIVES<br/>[FR] DERIVES DE QUINAZOLINE申请人:ASTRAZENECA AB公开号:WO2005051923A1公开(公告)日:2005-06-09A quinazoline derivative of the Formula (I): wherein the substituents are as defined in the text for use in the production of an anti proliferative effect which effect is produced alone or in part by inhibiting erbB2 receptor tyrosine kinase in a warm-blooded animal such as man.
表征谱图
-
氢谱1HNMR
-
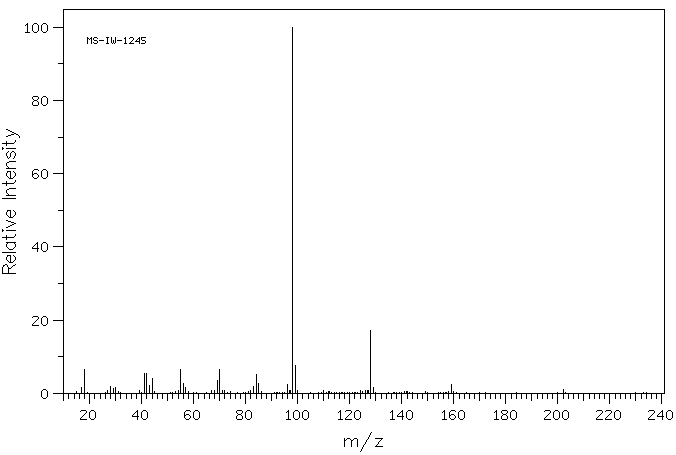
质谱MS
-
碳谱13CNMR
-
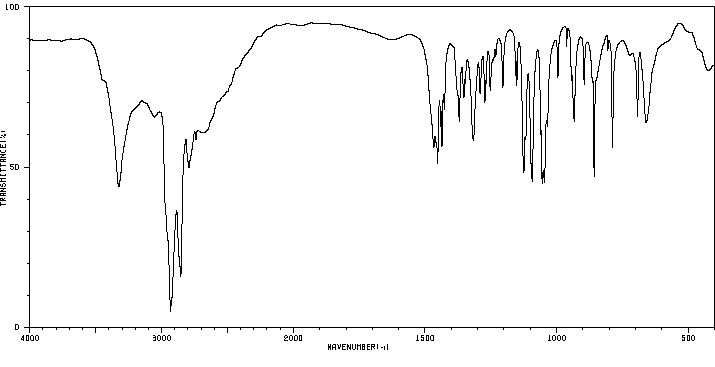
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(R)-3-甲基哌啶盐酸盐;
(R)-2-苄基哌啶-1-羧酸叔丁酯
((3S,4R)-3-氨基-4-羟基哌啶-1-基)(2-(1-(环丙基甲基)-1H-吲哚-2-基)-7-甲氧基-1-甲基-1H-苯并[d]咪唑-5-基)甲酮盐酸盐
高氯酸哌啶
高托品酮肟
马来酸帕罗西汀
颜料红48:4
顺式3-氟哌啶-4-醇盐酸盐
顺式2,6-二甲基哌啶-4-酮
顺式1-苄基-4-甲基-3-甲氨基-哌啶
顺式-叔丁基4-羟基-3-甲基哌啶-1-羧酸酯
顺式-6-甲基-哌啶-1,3-二甲酸1-叔丁酯
顺式-5-(三氟甲基)哌啶-3-羧酸甲酯盐酸盐
顺式-4-叔丁基-2-甲基哌啶
顺式-4-Boc-氨基哌啶-3-甲酸甲酯
顺式-4-(氮杂环丁烷-1-基)-3-氟哌
顺式-3-顺式-4-氨基哌啶
顺式-3-甲氧基-4-氨基哌啶
顺式-3-BOC-3,7-二氮杂双环[4.2.0]辛烷
顺式-3-(1-吡咯烷基)环丁腈
顺式-3,5-哌啶二羧酸
顺式-3,4-二溴-3-甲基吡咯烷盐酸盐
顺式-2,6-二甲基-4-氧代哌啶-1-羧酸叔丁基酯
顺式-1-叔丁氧羰基-4-甲基氨基-3-羟基哌啶
顺式-1-boc-3,4-二氨基哌啶
顺式-1-(4-叔丁基环己基)-4-苯基-4-哌啶腈
顺式-1,3-二甲基-4-乙炔基-6-苯基-3,4-哌啶二醇
顺-4-(4-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-4-(2-氟苯基)-1-(4-异丙基环己基)-4-哌啶羧酸
顺-3-氨基-4-氟哌啶-1-羧酸叔丁酯
顺-1-苄基-4-甲基哌啶-3-氨基酸甲酯盐酸盐
非莫西汀
雷芬那辛
雷拉地尔
阿维巴坦中间体4
阿格列汀杂质
阿尼利定盐酸盐 CII
阿尼利定
阿塔匹酮
阿哌沙班杂质BMS-591455
阿哌沙班杂质87
阿哌沙班杂质52
阿哌沙班杂质51
阿哌沙班杂质5
阿哌沙班杂质
阿哌沙班杂质
阿哌沙班-d3
阿哌沙班
阻聚剂701
间氨基谷氨酰胺