(Z)-2-methyl-2-penetenal | 16958-22-8
中文名称
——
中文别名
——
英文名称
(Z)-2-methyl-2-penetenal
英文别名
(E)-2-methyl-2-pentenal;(Z)-2-methylpent-2-enal;(Z)-2-methyl-pent-2-enal;cis-2-Methyl-2-pentenal;2-Methyl-pent-2-enal;Methyl-2-pentenal;2-Methyl-2-pentenal, (2Z)-
CAS
16958-22-8
化学式
C6H10O
mdl
——
分子量
98.1448
InChiKey
IDEYZABHVQLHAF-XQRVVYSFSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:138.5-139.6 °C(Press: 764 Torr)
-
密度:0.8536 g/cm3
计算性质
-
辛醇/水分配系数(LogP):1.4
-
重原子数:7
-
可旋转键数:2
-
环数:0.0
-
sp3杂化的碳原子比例:0.5
-
拓扑面积:17.1
-
氢给体数:0
-
氢受体数:1
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2-甲基-2-戊烯醛 (E)-2-methylpent-2-enal 14250-96-5 C6H10O 98.1448 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— (E)-2-methyl-2-pentenoyl chloride 55764-37-9 C6H9ClO 132.59
反应信息
-
作为反应物:描述:参考文献:名称:KASAXARA, AKIRA;IDZUMI, TAEHKO摘要:DOI:
-
作为产物:描述:2-甲基-2-戊烯醛 在 [Cp*Ru(cod)Cl][BF4] 、 氢气 、 potassium acetate 、 potassium carbonate 、 戴斯-马丁氧化剂 、 三乙胺 、 fumaric acid 作用下, 以 甲醇 、 二氯甲烷 、 丙酮 为溶剂, 20.0~80.0 ℃ 、500.01 kPa 条件下, 反应 21.67h, 生成 (Z)-2-methyl-2-penetenal参考文献:名称:二烯醇酯的选择性1,4-氢化加氢合成(Z)-三取代的烯丙醇:(-)-β-檀香醇的改进合成摘要:(E)-三取代的烯丙醇通常由相应的(E)-烯醛制备,它们本身可以通过简单的醛醇缩合反应容易地获得。我们证明,通过钌催化的相应的二烯醇乙酸酯的1,4-加氢反应,可以将这些非常相同的(E)-烯醛转化为(Z)-三取代的烯丙基乙酸酯(因此也可以是醇)。这种解决长期存在问题的简单方法已应用于工业上可行的(-)-β-檀香醇合成。DOI:10.1002/chem.201002729
文献信息
-
Rapid Organocatalytic Aldehyde-Aldehyde Condensation Reactions作者:Anniina Erkkilä、Petri M. PihkoDOI:10.1002/ejoc.200700292日期:2007.9We report the results of the systematic optimization of the α-methylenation of aldehydes with aqueous formaldehyde. A simple combination of a secondary amine catalyst and a weak acid co-catalyst has been identified, allowing access to α-substituted acroleins in a matter of minutes. In the absence of formaldehyde, the catalytic system promoted the self-condensation reaction of α,β-unsaturated aldehydes
-
NMR Investigations on the Proline-Catalyzed Aldehyde Self-Condensation: Mannich Mechanism, Dienamine Detection, and Erosion of the Aldol Addition Selectivity作者:Markus B. Schmid、Kirsten Zeitler、Ruth M. GschwindDOI:10.1021/jo200431v日期:2011.5.6The proline-catalyzed self-condensation of aliphatic aldehydes in DMSO with varying amounts of catalyst was studied by in situ NMR spectroscopy. The reaction profiles and intermediates observed as well as deuteration studies reveal that the proline-catalyzed aldol addition and condensation are competing, but not consecutive, reaction pathways. In addition, the rate-determining step of the condensation
-
Godfroid,J.-J., Bulletin de la Societe Chimique de France, 1964, p. 2929 - 2943作者:Godfroid,J.-J.DOI:——日期:——
-
Casiraghi, Giovanni; Casnati, Giuseppe; Pochini, Andrea, Journal of the Chemical Society. Perkin transactions I, 1982, p. 805 - 808作者:Casiraghi, Giovanni、Casnati, Giuseppe、Pochini, Andrea、Ungaro, RoccoDOI:——日期:——
-
Reaction of Chloromethylcarbene with Trimethylsilyl Enol Ethers; Preparation of α-Methylene Ketones and α-Methyl-α,β-unsaturated Ketones and Aldehydes作者:Luis Blanco、Philippe Amice、Jean-Marie ConiaDOI:10.1055/s-1981-29421日期:——
表征谱图
-
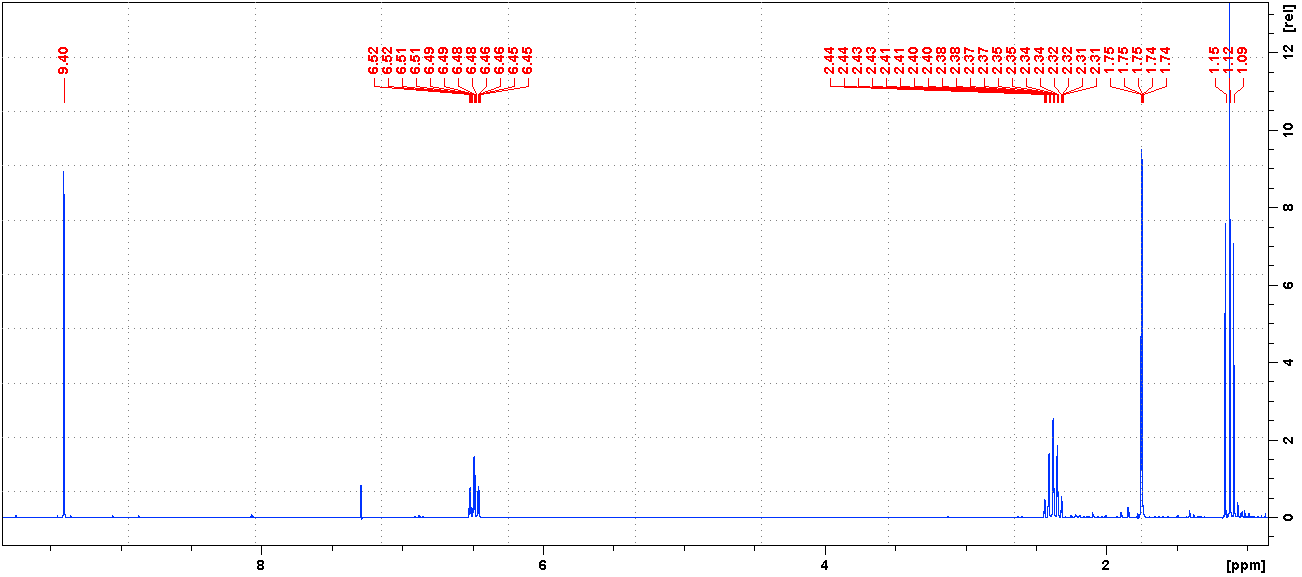
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷