1-溴蒽醌 | 632-83-7
中文名称
1-溴蒽醌
中文别名
1-溴代蒽醌;1-溴-9,10-二酮
英文名称
1-Bromoanthraquinone
英文别名
1-bromoanthracene-9,10-dione
CAS
632-83-7
化学式
C14H7BrO2
mdl
MFCD00451689
分子量
287.112
InChiKey
CXTPIHZYOGDSLV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:191 °C
-
沸点:444.9±34.0 °C(Predicted)
-
密度:1.5425 (rough estimate)
-
稳定性/保质期:
远离氧化物。
计算性质
-
辛醇/水分配系数(LogP):4
-
重原子数:17
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:34.1
-
氢给体数:0
-
氢受体数:2
安全信息
-
储存条件:存放在密封容器内,并置于阴凉、干燥处。请将储藏地点远离氧化剂和火源。
SDS
1-溴蒽醌 修改号码:5
模块 1. 化学品
产品名称: 1-BromoaNThraquinone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-溴蒽醌
百分比: >95.0%(GC)
CAS编码: 632-83-7
分子式: C14H7BrO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1-溴蒽醌 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-黄红色
气味: 无资料
pH: 无数据资料
熔点:
191°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-溴蒽醌 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-溴蒽醌 修改号码:5
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 1-BromoaNThraquinone
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害 未分类
环境危害 未分类
GHS标签元素
图标或危害标志 无
信号词 无信号词
危险描述 无
防范说明 无
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 1-溴蒽醌
百分比: >95.0%(GC)
CAS编码: 632-83-7
分子式: C14H7BrO2
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适请求医/就诊。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,求医/就诊。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
1-溴蒽醌 修改号码:5
模块 5. 消防措施
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 极淡的黄色-黄红色
气味: 无资料
pH: 无数据资料
熔点:
191°C
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂] 无资料
1-溴蒽醌 修改号码:5
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 溴化氢
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
1-溴蒽醌 修改号码:5
模块16 - 其他信息
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 1,5-二溴蒽醌 1,5-dibromo-9,10-anthraquinone 602-77-7 C14H6Br2O2 366.008 蒽醌 9,10-phenanthrenequinone 84-65-1 C14H8O2 208.216 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-bromoanthrone 87666-45-3 C14H9BrO 273.129 2-溴蒽醌 2-bromoanthracene-9,10-dione 572-83-8 C14H7BrO2 287.112 1-氨基-4-溴蒽醌 1-amino-4-bromo-9,10-anthracenedione 81-62-9 C14H8BrNO2 302.127 蒽醌 9,10-phenanthrenequinone 84-65-1 C14H8O2 208.216 —— 1-bromo-4-nitro-anthraquinone 780038-86-0 C14H6BrNO4 332.11
文献信息
-
SILANE COUPLING COMPOUNDS AND MEDICAL AND/OR DENTAL CURABLE COMPOSITIONS COMPRISING THE SAME申请人:KABUSHIKI KAISHA SHOFU公开号:US20190300552A1公开(公告)日:2019-10-03The present invention relate to a novel silane coupling agent and a medical and/or dental curable composition comprising the same. It is an object of the present invention to provide a novel silane coupling agent that imparts high affinity to a radical polymerizable monomer, thereby imparting high mechanical strength, flexibility and durability when used for a medical and/or dental curable composition, and an inorganic filler surface-treated with the novel silane coupling agent and a novel medical and/or dental curable composition. A silane coupling agent including repeating units such as a urethane bond and polyethylene glycol (ether bond) at a specific position is used.
-
Radical Alkyne <i>peri</i> ‐Annulation Reactions for the Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene作者:Nikolay P. Tsvetkov、Edgar Gonzalez‐Rodriguez、Audrey Hughes、Gabriel dos Passos Gomes、Frankie D. White、Febin Kuriakose、Igor V. AlabuginDOI:10.1002/anie.201712783日期:2018.3.26cyclization reactions at a peri position were used for the synthesis of polyaromatic compounds. Depending on the choice of reaction conditions and substrate, this flexible approach led to Bu3Sn‐substituted phenalene, benzanthrene, and olympicene derivatives. Subsequent reactions with electrophiles provided synthetic access to previously inaccessible functionalized polyaromatic compounds.
-
DENTAL ADHESIVE申请人:KURARAY NORITAKE DENTAL INC.公开号:US20170196778A1公开(公告)日:2017-07-13The present invention provides a dental adhesive exhibiting excellent initial bond strength and bond durability to both enamel and dentin. The present invention relates to a dental adhesive containing: an asymmetric acrylamide-methacrylic acid ester compound (a); an acid group-containing (meth)acrylic polymerizable monomer (b); and a water-soluble polymerizable monomer (c). The asymmetric acrylamide-methacrylic acid ester compound (a) is represented by the following general formula (1): where X is an optionally substituted, linear or branched C 1 to C 6 aliphatic group or an optionally substituted aromatic group, the aliphatic group is optionally interrupted by at least one linking group selected from the group consisting of —O—, —S—, —CO—, —CO—O—, —O—CO—, —NR 1 —, —CO—NR 1 —, —NR 1 —CO—, —CO—O—NR 1 —, —O—CO—NR 1 —, and —NR 1 —CO—NR 1 —, and R 1 is a hydrogen atom or an optionally substituted, linear or branched C 1 to C 6 aliphatic group.本发明提供了一种牙科粘接剂,具有优异的初始粘接强度和对牙釉质和牙本质的粘接耐久性。本发明涉及一种含有以下成分的牙科粘接剂:不对称丙烯酰胺-甲基丙烯酸酯化合物(a);含酸基的(甲基)丙烯酸聚合单体(b);以及水溶性聚合单体(c)。不对称丙烯酰胺-甲基丙烯酸酯化合物(a)由下述一般式(1)表示:其中X是可选择取代的线性或支链状的C1到C6脂肪族基或可选择取代的芳香族基,所述脂肪族基可被来自由—O—、—S—、—CO—、—CO—O—、—O—CO—、—NR1—、—CO—NR1—、—NR1—CO—、—CO—O—NR1—、—O—CO—NR1—和—NR1—CO—NR1—组成的至少一个连接基中断,R1为氢原子或可选择取代的线性或支链状的C1到C6脂肪族基。
-
Restricted Rotation Involving the Tetrahedral Carbon. IL. Singly<i>peri</i>-Substituted 9-Isopropyltriptycenes Revisited作者:Gaku Yamamoto、Michinori OkiDOI:10.1246/bcsj.56.2082日期:1983.79-Isopropyltriptycene derivatives carrying a fluoro, chloro, bromo, or t-butyl substituent in a peri-position were synthesized and their dynamic NMR behavior was studied to see the dependence of the barrier to rotation of the bridgehead substituent upon the bulkiness of the peri-substituent. The data obtained in this work together with those reported earlier disclose that, among the derivatives in which the peri-substituent is not buttressed by the 2-substituent, the peri-methoxy compound has the highest barrier and the barrier decreases as the bulkiness of the peri-substituent increases. 1,2,3,4-Tetrachloro and tetrabromo derivatives show the slightly positive buttressing effect.
-
一种化合物及包含其的有机光电装置申请人:上海天马有机发光显示技术有限公司公开号:CN111499618A公开(公告)日:2020-08-07本发明提供了一种化合物及包含其的有机光电装置。所述化合物具有式I所示结构。所述有机光电装置包括阳极、阴极,以及位于所述阳极和阴极之间的至少一层有机薄膜层;所述至少一层有机薄膜层选自空穴注入层、空穴传输层、电子阻挡层、发光层、空穴阻挡层、电子传输层和电子注入层中的一种或至少两种的组合,且包括发光层;至少一层所述有机薄膜层中含有至少一种上述化合物。本发明提供的化合物分子具有较高的刚性和大的共轭体系,具有较深的LUMO能级和较高的三线态能级,且分子稳定性好,不易结晶,可用作有机光电装置的电子传输材料或空穴阻挡材料,有助于降低器件的启亮电压,提高电流效率,提高寿命。
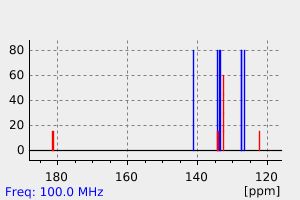
表征谱图
-
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
齐斯托醌
黄决明素
马普替林相关物质D
马普替林杂质E(N-甲基马普替林)
马普替林杂质D
马普替林D3
马普替林
颜料黄199
颜料黄147
颜料黄123
颜料黄108
颜料红89
颜料红85
颜料红251
颜料红177
颜料紫27
顺式-1-(9-蒽基)-2-硝基乙烯
阿美蒽醌
阳离子蓝FGL
阳离子蓝3RL
长蠕孢素
镁蒽四氢呋喃络合物
镁蒽
锈色洋地黄醌醇
锂钠2-[[4-[[3-[(4-氨基-9,10-二氧代-3-磺基-1-蒽基)氨基]-2,2-二甲基-丙基]氨基]-6-氯-1,3,5-三嗪-2-基]氨基]苯-1,4-二磺酸酯
锂胭脂红
链蠕孢素
铷离子载体I
铝洋红
铂(2+)二氯化1-({2-[(2-氨基乙基)氨基]乙基}氨基)蒽-9,10-二酮(1:1)
钾6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠alpha-(丙烯酰氨基)-[4-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]苯氧基]甲苯磺酸盐
钠[[3-[[4-(环己基氨基)-9,10-二氢-9,10-二氧代-1-蒽基]氨基]-1-氧代丙基]氨基]苯磺酸盐
钠[3-[[9,10-二氢-4-(异丙基氨基)-9,10-二氧代-1-蒽基]氨基]丁基]苯磺酸盐
钠6,11-二氧代-6,11-二氢-1H-蒽并[1,2-d][1,2,3]三唑-4-磺酸酯
钠4-({4-[乙酰基(乙基)氨基]苯基}氨基)-1-氨基-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠2-[(4-氨基-9,10-二氧代-3-磺基-9,10-二氢-1-蒽基)氨基]-4-{[2-(磺基氧基)乙基]磺酰基}苯甲酸酯
钠1-氨基-9,10-二氢-4-[[4-(1,1-二甲基乙基)-2-甲基苯基]氨基]-9,10-二氧代蒽-2-磺酸盐
钠1-氨基-4-[(3-{[(4-甲基苯基)磺酰基]氨基}苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-[(3,4-二甲基苯基)氨基]-9,10-二氧代-9,10-二氢-2-蒽磺酸酯
钠1-氨基-4-(1,3-苯并噻唑-2-基硫基)-9,10-二氧代蒽-2-磺酸盐
醌茜隐色体
醌茜素
酸性蓝P-RLS
酸性蓝41
酸性蓝27
酸性蓝127:1
酸性紫48
酸性紫43
酸性兰62