N-(苯磺酰基)-N-甲基苯磺酰胺 | 2532-06-1
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:110-111 °C
-
沸点:475.7±28.0 °C(Predicted)
-
密度:1.386±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:20
-
可旋转键数:4
-
环数:2.0
-
sp3杂化的碳原子比例:0.08
-
拓扑面积:88.3
-
氢给体数:0
-
氢受体数:5
SDS
上下游信息
反应信息
-
作为反应物:描述:N-(苯磺酰基)-N-甲基苯磺酰胺 、 4-乙酰基苯硼酸 在 potassium phosphate 、 1,3-bis[(diphenylphosphino)propane]dichloronickel(II) 作用下, 以 1,4-二氧六环 为溶剂, 以28%的产率得到联苯单乙酮参考文献:名称:N,N-二磺酰甲胺和芳基硼酸的镍催化脱硫 Suzuki-Miyaura 交叉偶联摘要:已开发出一种用于从 N,N-二磺酰基甲胺和芳基硼酸合成联芳基化合物的镍催化方法。代替芳烃磺酰氯,使用各种 N,N-二磺酰甲胺作为芳基源,通过 SO2 的挤出得到中等至良好产率的交叉偶联产物。DOI:10.1002/ejoc.201402919
-
作为产物:描述:参考文献:名称:烷基的研究?通过还原消除高氧化态单体钯配合物形成氮键摘要:的一系列定义的钯(II)配合物带有二齿配体,和一个甲基和一个amidato取代基是用目的进行氧化,以便更好地的对C内在要求理解 N键-形成选自Pd络合物在高氧化态。这项工作阐明了各个氮源的作用,并且对Pd催化的烷基氮键形成反应具有重要意义。DOI:10.1002/hlca.201200430
-
作为试剂:描述:1-BOC-4-甲磺酰基氧甲基哌啶 在 (1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride 、 N-(苯磺酰基)-N-甲基苯磺酰胺 、 sodium hexamethyldisilazane 、 sodium carbonate 、 caesium carbonate 、 间氯过氧苯甲酸 作用下, 以 四氢呋喃 、 1,4-二氧六环 、 二氯甲烷 、 水 、 丙酮 为溶剂, 反应 49.3h, 生成 tert-butyl 4-(((2',4'-difluoro-[1,1'-biphenyl]-4-yl)sulfonyl)difluoromethyl)piperidine-1-carboxylate参考文献:名称:[EN] N-ACYL-{4-[(4-ARYL-PHENYL)SULFONYLMETHYL]PIPERIDINE} COMPOUNDS AND THEIR THERAPEUTIC USE
[FR] COMPOSÉS N-ACYL-{4-[(4-ARYL-PHÉNYL)SULFONYLMÉTHYL]PIPÉRIDINE} ET LEUR UTILISATION THÉRAPEUTIQUE摘要:本发明一般涉及治疗化合物领域。更具体地,本发明涉及以下公式的某些N-酰基-{4-[(4-芳基苯基)磺酰甲基]哌啶}化合物(以下统称为NASMP化合物),这些化合物可用于治疗包括多发性骨髓瘤、弥漫性大B细胞淋巴瘤、急性髓细胞白血病、嗜酸性白血病、胶质母细胞瘤、黑色素瘤、卵巢癌、化疗耐药癌症、放射治疗耐药癌症、炎症性关节炎、类风湿关节炎、银屑病性关节炎、牛皮癣、溃疡性结肠炎、克罗恩病、系统性红斑狼疮(SLE)、狼疮性肾炎、哮喘、慢性阻塞性肺病(COPD)、汗腺脓肿、自身免疫性肝炎等疾病的治疗,本发明还涉及包含这些化合物的药物组合物,以及这些化合物和组合物在治疗中的使用。公开号:WO2020212581A1
文献信息
-
Lewis acid-assisted N-fluorobenzenesulfonimide-based electrophilic fluorine catalysis in Beckmann rearrangement作者:Fukai Xie、Chuan Du、Yadong Pang、Xu Lian、Chentao Xue、Yanyu Chen、Xuefei Wang、Maosheng Cheng、Chun Guo、Bin Lin、Yongxiang LiuDOI:10.1016/j.tetlet.2016.11.054日期:2016.12A microwave-assisted N-fluorobenzenesulfonimide (NFSI)/Lewis acid-catalyzed Beckmann rearrangement was developed. The remarkable promotion to the electrophilicity of NFSI by Lewis acids was illustrated utilizing a series of readily available oxime substrates. The action model between NFSI and Lewis acids was probed by control experiments and theoretical calculations.
-
Electrophilic fluorination of N,N-dimethylaniline, N,N-dimethylnaphthalen-1-amine and 1,8-bis(dimethylamino)naphthalene with N–F reagents作者:Vladimir I. Sorokin、Alexander F. Pozharskii、Valery A. OzeryanskiiDOI:10.1016/j.jfluchem.2013.06.017日期:2013.10Reaction of N,N-dimethylaniline, N,N-dimethylnaphthalen-1-amine and 1,8-bis(dimethylamino)naphthalene (proton sponge) with 1-chloromethyl-4-fluorodiazonia-bicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor) and N-fluorobenzenesulfonimide (NFSI) has been studied under various conditions. Unlike the proton sponge, which is fluorinated rather selectively at the ortho-position to NMe2 group, producing的反应Ñ,Ñ二甲基苯胺,Ñ,Ñ -dimethylnaphthalen -1-胺和1,8-双(二甲基氨基)萘(质子海绵)与1-氯甲基-4- fluorodiazonia -二环[2.2.2]辛烷二(四氟硼酸盐)(Selectfluor)和N-氟苯磺酰亚胺(NFSI)已在各种条件下进行了研究。与质子海绵不同,质子海绵在NMe 2基团的邻位上选择性地氟化,以中等收率生成2-氟衍生物,而另外两种胺与Selectfluor和NFSI反应,具有很强的去污能力,并形成了相应的联芳基的复杂混合物,联芳基甲烷和N-去甲基化的产品。还少量形成了2-氟和4-氟衍生物,其中主要是前一种异构体。使用NFSI–ZrCl 4系统可导致芳香环的竞争性氯化反应。
-
[EN] TRANSITION METAL-CATALYZED IMIDATION OF ARENES<br/>[FR] IMIDATION D'ARÈNES CATALYSÉE PAR DES MÉTAUX DE TRANSITION申请人:HARVARD COLLEGE公开号:WO2015031725A1公开(公告)日:2015-03-05The present invention provides novel transition metal complexes (e.g., complexes of any one of Formulae (C1) to (C25)) that include an amine-N-oxide motif. The invention also provides methods of using these transition metal complexes in preparing N-aryl or N- heteroaryl sulfonimides (e.g., compounds of Formula (I)) and aryl or heteroaryl amines (e.g., compounds of Formula (II)). The inventive methods involve imidation of arenes and heteroarenes (e.g., compounds of Formula (A)) using an imidating agent (e.g., a compound of Formula (B), such as N-fluorobenzenesulfonimide (NFBS or NFSI)) in the presence of a single-electron reductant (e.g., an Ag(I) or Ru(II) salt).
-
Polysulfonylamine, LXXVI [1] Zwei supramolekulare Isomere im gleichen Kristall: Darstellung und Struktur des monomeren 18-Krone-6-Komplexes (CH<sub>2</sub>CH<sub>2</sub>O)<sub>6</sub>·2 MeN(SO<sub>2</sub>Me)(SO<sub>2</sub>Ph) / Polysulfonylamines, LXXVI [1] Occurrence of Two Supramolecular Isomers in One Crystal: Synthesis and Structure of the Monomeric 18-Crown-6 Complex (CH<sub>2</sub>CH<sub>2</sub>O)<sub>6</sub> • 2 MeN(SO<sub>2</sub>Me) (SO<sub>2</sub>Ph)作者:Dagmar Henschel、Oliver Hiemisch、Armand Blaschette、Peter G. JonesDOI:10.1515/znb-1996-0916日期:1996.9.1
The title complex, obtained by co-crystallization of its molecular components from methanol, was characterized by low-temperature X-ray diffraction. The crystal structure (triclinic, space group P1̄, Z = 2) consists of two monomeric and crystallographically centrosymmetric formula units A and B which may be classified as supramolecular stereo- and/or linkage isomers. In both units, the receptor rings adopt the frequently observed D3d pseudosymmetry, and the symmetry-related substrate molecules are situated above and below the polyether rings. The isomerism of the supermolecules arises from markedly different confomations of their substrate components. In B, the methyl groups display a transoid orientation (torsional angle for C - N - S - C : 135.5°) and the N-methyldisulfonylamine is solely attached by three S - C - H ··· O interactions to a set of three alternating crown oxygen atoms. In isomer A, the C -N - S - Cmethyl sequence is cisoid (torsional angle: 83.1°) and the substrate is connected to the crown ether by three S - C - H ··· O bonds and an additional N - C - H ··· O interaction. The H ··· O distances and C -H ··· O angles of the seven independent hydrogen bonds lie within the intervals 230-260 pm and 140-180°, respectively. A supermolecule involving MeN (SO2Ph)2 and 18-crown-6 could not be isolated, whereas the binary complex (CH2CH2O)6•2 MeN(SO2F)2 is readily obtained by co-crystallization of the components. The latter complex showed severe disorder of the N(SO2F)2 moieties and its structure could not be refined satisfactorily.
通过在甲醇中共结晶其分子组分得到的标题配合物,通过低温X射线衍射进行了表征。晶体结构(三斜晶系,空间群P1̄,Z = 2)由两个单体和晶体学中心对称的式单位A和B组成,可归类为超分子立体和/或连接异构体。在两个单元中,受体环采用了常见的D3d伪对称性,并且对称相关的底物分子位于聚醚环的上方和下方。超分子的异构性来自于其底物组分明显不同的构象。在B中,甲基基团显示出反式定向(C-N-S-C的扭转角:135.5°),N-甲基二磺酰胺仅通过三个S-C-H···O相互作用连接到一组三个交替的冠醚氧原子。在异构体A中,C-N-S-Cmethyl序列为顺式(扭转角:83.1°),底物通过三个S-C-H···O键和一个额外的N-C-H···O相互作用连接到冠醚中。七个独立氢键的H···O距离和C-H···O角度分别位于230-260 pm和140-180°的区间内。无法分离包含MeN(SO2Ph)2和18-冠-6的超分子,而二元复合物(CH2CH2O)6•2 MeN(SO2F)2可以通过组分的共结晶轻松获得。后者复合物的N(SO2F)2部分存在严重的无序性,其结构无法得到令人满意的精细。 -
[EN] SYNTHESIS OF FLUORINATED NUCLEOTIDES<br/>[FR] SYNTHÈSE DE NUCLÉOTIDES FLUORÉS申请人:MERCK SHARP & DOHME公开号:WO2022035917A1公开(公告)日:2022-02-17The present invention relates to efficient processes useful in the preparation of fluorinated nucleosides, such as (O-[(2R,3R,4S,5R)-5-(6-amino-9H-purin-9-yl)-4-fluoro-3-hydroxyoxolan-2-yl]methyl}O,O-dihydrogen phosphorothioate, also known as 2'-(S)-fluoro-thio-adenosine monophosphate or 2'-F-thio-AMP. Such fluorinated nucleosides may be useful as a biologically active compound and or as an intermediate for the synthesis of more complex biologically active compounds. The present invention also encompasses intermediates useful in the disclosed synthetic processes and the methods of their preparation.
表征谱图
-
氢谱1HNMR
-
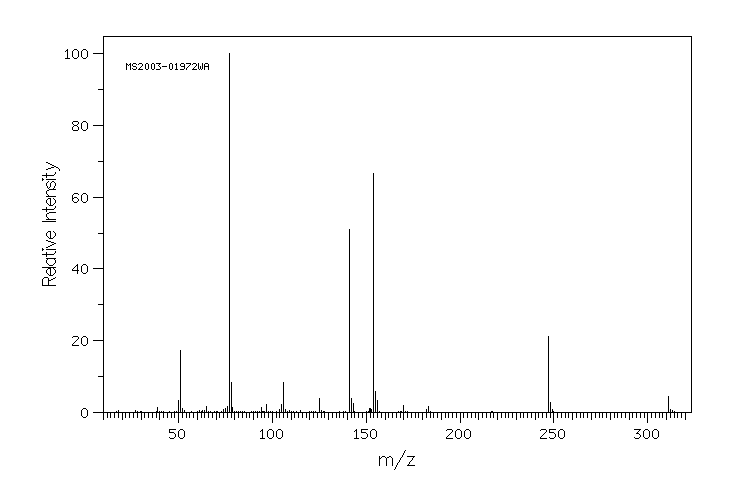
质谱MS
-
碳谱13CNMR
-
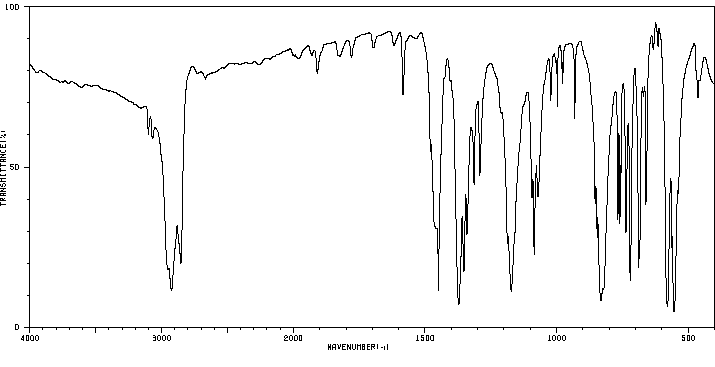
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息