2,5-二溴间二甲苯 | 100189-84-2
中文名称
2,5-二溴间二甲苯
中文别名
2,4-二氯苯胺氯化物
英文名称
2,5-dibromo-1,3-dimethylbenzene
英文别名
2,5-dibromo-m-xylene;2,6-dimethyl-1,4-dibromobenzene;1,4-dibromo-2,6-dimethylbenzene
CAS
100189-84-2
化学式
C8H8Br2
mdl
——
分子量
263.96
InChiKey
XXFGKGMZLZIPCN-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:261.7±35.0 °C(Predicted)
-
密度:1.710±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):4.8
-
重原子数:10
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.25
-
拓扑面积:0
-
氢给体数:0
-
氢受体数:0
安全信息
-
海关编码:2903999090
-
危险性防范说明:P261,P280,P305+P351+P338
-
危险性描述:H302+H312+H332,H315,H319,H335
-
储存条件:室温且干燥
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2,5-Dibromo-m-xylene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,5-Dibromo-m-xylene
CAS number: 100189-84-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Br2
Molecular weight: 264.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2,5-Dibromo-m-xylene
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2,5-Dibromo-m-xylene
CAS number: 100189-84-2
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C8H8Br2
Molecular weight: 264.0
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, hydrogen bromide.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,6-二甲基溴苯 2-Bromo-m-xylene 576-22-7 C8H9Br 185.063 4-溴-3,5-二甲基苯胺 4-bromo-3,5-dimethylaniline 59557-90-3 C8H10BrN 200.078 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 2,5-dibromo-1,3-bis(bromomethyl)benzene 689260-65-9 C8H6Br4 421.752 3,5-二甲基-4-溴苯腈 4-bromo-3,5-dimethylbenzonitrile 75344-77-3 C9H8BrN 210.073 4-溴-3,5-二甲基苯甲醛 4-bromo-3,5-dimethylbenzaldehyde 400822-47-1 C9H9BrO 213.074 4-溴-3,5-二甲基苄醇 (4-bromo-3,5-dimethylphenyl)methanol 27006-02-6 C9H11BrO 215.09 —— 2,5-dibromo-1,3-bis(cyanomethyl)benzene 740815-82-1 C10H6Br2N2 313.979 —— 4-bromo-3,5-dimethylbenzaldehyde oxime 400822-49-3 C9H10BrNO 228.088
反应信息
-
作为反应物:描述:参考文献:名称:含用于染料敏化太阳能电池的辅助苯并三唑受体的卟啉敏化剂:空间位阻和共敏化的影响摘要:染料敏化太阳能电池(DSSC)在开发用于利用太阳能的光伏设备中引起了广泛的关注。对于开发全色和有效的卟啉敏化剂,已证明是引入吸电子苯并噻二唑单元作为额外电子受体的有效方法。相反,在这方面,结构相似的苯并三唑部分仍然相对未知。在这项工作中,我们合成了一种新型的卟啉染料,其中含有一个额外的苯并三唑电子受体。光物理和电化学研究表明,苯并三唑单元会引起红移吸收和较窄的带隙。因此,J sc为17.00 mA cm -2时,已达到8.39%的合理效率。和V OC 712毫伏。在此基础上,为了研究苯并三唑与相邻的亚苯基环之间的二面角的影响,将一个或两个甲基连接至亚苯基环的邻位。结果,增大的扭转角在染料的LUMO和TiO 2之间引起不良的电子耦合。,导致效率分别降低了6.61%和3.62%。为了进一步提高效率,采用了共吸附和共敏化方法。效率已分别成功提升至9.32%,8.49%和7.34%。这些结果证明了DOI:10.1016/j.dyepig.2018.03.054
-
作为产物:描述:2,6-二甲基溴苯 在 (1,5-cyclooctadiene)(methoxy)iridium(I) dimer 、 联硼酸频那醇酯 、 4,4'-二叔丁基-2,2'-二吡啶 、 copper(ll) bromide 作用下, 以 正己烷 、 甲醇 为溶剂, 反应 120.0h, 以91%的产率得到2,5-二溴间二甲苯参考文献:名称:四阳离子双三芳基硼烷 1,3-丁二炔作为组合荧光和拉曼探针,用于同时和选择性传感各种 DNA、RNA 和蛋白质。摘要:双三芳基硼烷四阳离子 (4-Ar2 B-3,5-Me2 C6 H2 )-C≡CC≡C-(3,5-Me2 C6 H2 -4-BAr2 [Ar=(2,6-Me2 -4- NMe3 -C6 H2 )+ ] (24+ ) 在荧光响应中表现出与我们最近发表的双-三芳基硼烷 5-(4-Ar2 B-3,5-Me2 C6 H2 )-2,2'- 明显不同的行为(C4 H2 S)2 -5'-(3,5-Me2 C6 H2 -4-BAr2 ) (34+ ). 中性双三芳基硼烷前体 2 N 的单晶 X 射线衍射数据证实其为棒状哑铃结构,这对于 DNA/RNA 靶向以及 BSA 蛋白结合非常重要。DNA/RNA/BSA 的荧光滴定显示 24+ 具有非常强的亲和力,并表明连接两者的接头特性的重要性三芳基硼烷。使用丁二炔而不是联噻吩连接体会产生相反的发射效应(猝灭与增强),并且 24+ 与 BSA 的结合比 34+ 强 100DOI:10.1002/chem.201905328
文献信息
-
The Reaction of Dihalotetramethylbenzenes with Fuming Nitric Acid as a New Convenient Route to Some Dihalotrimethylbenzylic Compounds作者:Hitomi Suzuki、Kiyomi Nakamura、Michiko TakeshimaDOI:10.1246/bcsj.44.2248日期:1971.8the latter in somewhat greater amount. 5,6-Dihalo-1,2,3,4-tetramethylbenzene (dihaloprehnitene) gave 5,6-dihalo-2,3,4-trimethylbenzyl nitrate as the sole nitrooxylation product. The reaction affords a new convenient route to precursors of various polysubstituted benzylic compounds hitherto not easily obtained by ordinary methods. Physical properties of some dihalotrimethylbenzylic compounds (chloride研究了用发烟硝酸硝化三种异构二氯和二溴四甲基苯得到的产物。3,6-二卤-1,2,4,5-四甲基苯(二卤代脲)主要生成 3,6-二卤-2,4,5-三甲基苄基硝酸酯或 1,2-双(硝基氧甲基)-3,6-二卤- 4,5-二甲苯,取决于反应温度和硝化剂的用量。4,6-二卤-1,2,3,5-四甲基苯(二卤代异丁二烯)生成 3,5-二卤-2,4,6-三甲基苄基硝酸酯和 2,6-二卤-3,4,5-三甲基苄基的混合物硝酸盐,后者的量稍大。5,6-二卤-1,2,3,4-四甲基苯(二卤代戊二烯)得到5,6-二卤-2,3,4-三甲基苄基硝酸盐,作为唯一的硝基酰化产物。该反应为迄今为止通过普通方法不易获得的各种多取代苄基化合物的前体提供了新的便利途径。一些二卤代三甲基苄基化合物(氯化物、硝酸盐、醋酸盐、酒精和双苄基醚)的物理特性已被记录。
-
Novel Thiophene Derivatives as Spingosine-1-Phosphate-1 Receptor Agonists
-
Rational Design of a Near‐infrared Fluorescence Probe for Ca <sup>2+</sup> Based on Phosphorus‐substituted Rhodamines Utilizing Photoinduced Electron Transfer作者:Shodai Takahashi、Kenjiro Hanaoka、Yohei Okubo、Honami Echizen、Takayuki Ikeno、Toru Komatsu、Tasuku Ueno、Kenzo Hirose、Masamitsu Iino、Tetsuo Nagano、Yasuteru UranoDOI:10.1002/asia.201901689日期:2020.2.17the cytosol, in marked contrast to our previously reported Ca2+ far‐red to NIR fluorescence probe based on the SiR scaffold, CaSiR‐1 AM, which is mainly localized in lysosomes as well as cytosol in living cells. CaPR‐1 showed longer‐wavelength absorption and emission (up to 712 nm) than CaSiR‐1. The new probe was able to image Ca2+ at dendrites and spines in brain slices, and should be a useful tool近红外(NIR)区域(650–900 nm)的荧光成像可用于生物成像,因为在此范围内背景自发荧光低且组织穿透率高。此外,NIR荧光可用作多色成像的绿色和红色的互补色窗口。在这里,我们比较了硅和磷取代的罗丹明(SiRs和PRs)的光诱导电子转移(PeT)介导的荧光猝灭,以指导改进的远红外至NIR荧光染料的开发。一系列新合成的PR的密度泛函理论计算和光物理评估的结果证实,与SiRs相比,PRs的荧光更容易通过PeT猝灭。在此基础上,我们设计并合成了用于Ca 2+的NIR荧光探针,CaPR-1及其膜渗透性乙酰氧甲基衍生物CaPR-1AM分布在细胞质中,这与我们先前报道的基于SiR支架CaSiR-的Ca 2+远红外NIR荧光探针形成鲜明对比1 AM,主要位于溶酶体以及活细胞的胞浆中。CAPR-1显示出更长波长的吸收和发射(高达712纳米)比CaSiR-1 。新的探针能够在脑切片的树突和棘上成像Ca
-
IRIDIUM COMPLEX COMPOUND, COMPOSITION CONTAINING THE COMPOUND AND SOLVENT, ORGANIC ELECTROLUMINESCENT ELEMENT CONTAINING THE COMPOUND, DISPLAY DEVICE, AND ILLUMINATION DEVICE申请人:MITSUBISHI CHEMICAL CORPORATION公开号:US20200317706A1公开(公告)日:2020-10-08Provided is an iridium complex compound represented by formula (1) below. [Ir is an iridium atom. C 1 to C 6 are carbon atoms. N 1 and N 2 are nitrogen atoms. R 1 to R 4 are each a hydrogen atom or a substituent. a to d are maximum integer numbers of possible substituents on rings Cy 1 to Cy 4 , respectively. m and n are each 1 or 2, and m+n=3. The ring Cy 1 is represented by formula (2) or (2′) below. The ring Cy 3 is an aromatic or heteroaromatic ring including the carbon atoms C 4 and C 5 . The ring Cy 4 is a heteroaromatic ring including the carbon atom C 6 and the nitrogen atom N 2 .]
-
一种以氧杂蒽酮为核心的杂环化合物、制备方法及其应用
表征谱图
-
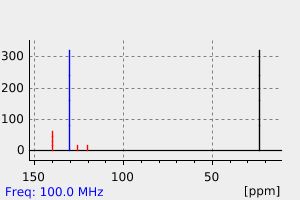
氢谱1HNMR
-
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫