5-叔丁基-2-甲基苯硫酚 | 7340-90-1
中文名称
5-叔丁基-2-甲基苯硫酚
中文别名
2-甲基-5-叔丁基噻吩;5-叔丁基-2-甲基硫代苯酚;5-叔丁基邻硫代甲酚;4-叔丁基-2-巯基甲苯
英文名称
2-methyl-5-tert-butylthiophenol
英文别名
5-tert-butyl-2-methylbenzenethiol;5-tert-butyl-2-methylthiophenol;2-methyl-5-tert-butylphenylthiol
CAS
7340-90-1
化学式
C11H16S
mdl
MFCD00142942
分子量
180.314
InChiKey
ZSBNXOIAJFVXMP-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:-10 °C
-
沸点:260 °C
-
密度:0.98
-
闪点:116°C
-
稳定性/保质期:
如果按照规格使用和储存,则不会分解。
计算性质
-
辛醇/水分配系数(LogP):4.1
-
重原子数:12
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:0.454
-
拓扑面积:1
-
氢给体数:1
-
氢受体数:1
安全信息
-
危险等级:9
-
安全说明:S26,S37/39,S61
-
危险类别码:R51/53,R36/38
-
海关编码:2930909090
-
包装等级:III
-
危险品运输编号:UN 3082
-
储存条件:密封保存在阴凉干燥处。
SDS
| Name: | 2-Methyl-5-tert-butylthiophenol 93% remainder isomer Material Safety Data Sheet |
| Synonym: | 5-(1,1-Dimethylethyl)-2-methylthiophenol; 2-Methyl-5-(1,1-dimethylethyl)thiopheno |
| CAS: | 7340-90-1 |
Synonym:5-(1,1-Dimethylethyl)-2-methylthiophenol; 2-Methyl-5-(1,1-dimethylethyl)thiopheno
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 7340-90-1 | 2-Methyl-5-tert-butylthiophenol | 93 | unlisted |
Risk Phrases: 36/38 51 53
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
Irritating to eyes and skin. Toxic to aquatic organisms. May cause long-term adverse effects in the aquatic environment.
Potential Health Effects
Eye:
Causes eye irritation. May cause chemical conjunctivitis.
Skin:
Causes skin irritation.
Ingestion:
May cause gastrointestinal irritation with nausea, vomiting and diarrhea.
Inhalation:
Causes respiratory tract irritation. Can produce delayed pulmonary edema.
Chronic:
Effects may be delayed.
Section 4 - FIRST AID MEASURES
Eyes: Immediately flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. Do NOT use mouth-to-mouth resuscitation.
Notes to Physician:
Treat symptomatically and supportively.
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Vapors may be heavier than air. They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Water may be ineffective. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Avoid contact with eyes, skin, and clothing. Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 7340-90-1: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
A respiratory protection program that meets OSHA's 29 CFR 1910.134 and ANSI Z88.2 requirements or European Standard EN 149 must be followed whenever workplace conditions warrant respirator use.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear, colorless
Odor: None reported.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: 260 deg C @ 760.00mm Hg
Freezing/Melting Point: -10 deg C
Autoignition Temperature: Not applicable.
Flash Point: 123 deg C ( 253.40 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature: Not available.
Solubility in water: Not available.
Specific Gravity/Density: .9800g/cm3
Molecular Formula: C11H16S
Molecular Weight: 180.31
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable at room temperature in closed containers under normal storage and handling conditions.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
No information found..
Hazardous Decomposition Products:
Carbon monoxide, oxides of sulfur, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported.
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 7340-90-1 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
2-Methyl-5-tert-butylthiophenol - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: XI
Risk Phrases:
R 36/38 Irritating to eyes and skin.
R 51 Toxic to aquatic organisms.
R 53 May cause long-term adverse effects in the
aquatic environment.
Safety Phrases:
S 26 In case of contact with eyes, rinse immediately
with plenty of water and seek medical advice.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37/39 Wear suitable gloves and eye/face
protection.
S 56 Do not discharge into drains or the
environment, dispose to an authorised waste collection
point.
WGK (Water Danger/Protection)
CAS# 7340-90-1: 2
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 7340-90-1 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 7340-90-1 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— bis(5-(tert-butyl)-2-methylphenyl)disulfide 34493-12-4 C22H30S2 358.612 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— bis(5-(tert-butyl)-2-methylphenyl)disulfide 34493-12-4 C22H30S2 358.612 —— 2-Methyl-5-t-butylbenzolsulfosaeure 25589-81-5 C11H16O3S 228.312 5-叔丁基-2-甲基苯磺酰胺 5-(tert-butyl)-2-methylbenzenesulfonamide 7155-00-2 C11H17NO2S 227.327
反应信息
-
作为反应物:描述:5-叔丁基-2-甲基苯硫酚 在 正丁基锂 、 草酰氯 、 三氟化硼乙醚 、 sodium methylate 、 sodium hydride 、 二异丁基氢化铝 、 二正丁基氧化锡 、 对甲苯磺酸 、 二甲基亚砜 作用下, 以 四氢呋喃 、 甲醇 、 正己烷 、 二氯甲烷 、 N,N-二甲基甲酰胺 、 甲苯 为溶剂, 反应 28.67h, 生成 (2-methyl-5-tert-butylphenyl) 2-O-benzyl-4-O-(p-methoxybenzyl)-3-O-(2-naphthalenyl-methyl)-7-S-tris(thiophenol)-l-glycero-1-thia-α-D-thiomannoheptopyranoside参考文献:名称:Improved methods for the stereoselective synthesis of mannoheptosyl donors and their glycosides: toward the synthesis of the trisaccharide repeating unit of the Campylobacter jejuni RM1221 capsular polysaccharide摘要:In view of the importance of 6-deoxymannoheptosides as structural units in the Campylobacter jejuni RM1221 capsular polysaccharide, the development of effective synthetic protocols for 4-O-6-S-alpha-cyanobenzylidene thio-o-mannoheptapyranoside donors carrying either 3-O-naphthylmethyl or 3-O-acetyl groups is described starting from D-mannose. In particular, tris(phenylthio)methyllithium is found to undergo highly stereoselective addition to a mannose-6-aldehyde in sharp contrast to the vinyl Grignard reagent whose reactions were essentially devoid of selectivity. A brief survey of coupling reactions with the two donors indicted the 3-O-acetyl system to be highly alpha-selective whereas the 3-O-naphthylmethyl congener was highly beta-selective indicating that the presence of the seventh carbon atom in these donors is not detrimental to coupling selectivity in either instance. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tet.2013.04.094
-
作为产物:描述:bis(5-(tert-butyl)-2-methylphenyl)disulfide 在 sodium tetrahydroborate 作用下, 生成 5-叔丁基-2-甲基苯硫酚参考文献:名称:硫基自由基促进的分子氧意外地催化了C = C键的裂解。摘要:在过渡金属催化剂和苯硫酚的促进下,分子氧对烯烃的氧化作用涉及将C = C键裂解为相应的羰基衍生物。在室温下,在过量的硫酚和催化剂(例如MnL(2)3a或VClL(2)3c)的存在下,该新反应在一种氧气气氛下于室温进行。它可用于芳族和脂族烯烃,以及官能化或非官能化的无环化合物,以高达98%的收率提供相应的酮和醛。通过一步制备芳香族(-)-4-乙酰基-1-甲基环己烯7e的一步制备方法可以说明这种催化氧化的合成兴趣,分离出的收率为73%。C = C键的断裂可能是由于β-氢过氧硫化物中间体12的催化分解而造成的,该中间体是通过在氧气存在下将硫酚自由基加成至烯烃而形成的。尽管使用了过量的硫酚,但仍将其转化为二硫化物,然后无需纯化即可将其还原,总收率为83%,从而可以回收利用。另外,通过二硫化物的光解产生的催化量的噻吩基可以促进在氧下的C = C键裂解。因此,在0.1当量的双(4-氯苯基)二硫化物4bDOI:10.1021/jo0013148
-
作为试剂:描述:盐酸文拉法辛 在 potassium carbonate 、 5-叔丁基-2-甲基苯硫酚 作用下, 以 polyethyleneglycol monomethylether 为溶剂, 反应 0.5h, 以98%的产率得到O-去甲文拉法辛参考文献:名称:[EN] METHOD OF PRODUCING 4-(2-(SUBSTITUTED)-1-(1-HYDROXYCYCLOHEXYL)ETHYL)PHENOLS BY O- DEMETHYLATION OF THEIR METHYLETHERS BY MEANS OF INODOROUS AROMATIC THIOLS
[FR] PROCÉDÉ DE PRÉPARATION DE PHÉNOLS-4-(1-(1-HYDROXYCYCLOHEXYL)-2-(SUBSTITUÉ)ÉTHYL) PAR O-DÉMÉTHYLATION DE LEURS ÉTHERS MÉTHYLIQUES AU MOYEN DE THIOLS AROMATIQUES INODORES摘要:公开号:WO2011124190A3
文献信息
-
An Organodiselenide with Dual Mimic Function of Sulfhydryl Oxidases and Glutathione Peroxidases: Aerial Oxidation of Organothiols to Organodisulfides作者:Vandana Rathore、Aditya Upadhyay、Sangit KumarDOI:10.1021/acs.orglett.8b02756日期:2018.10.5peroxidase (GPx) enzymes for oxidation of thiols by oxygen and hydrogen peroxide, respectively, into disulfides has been presented. The developed catalyst oxidizes an array of organothiols into respective disulfides in practical yields by using aerial O2 to avoid any reagents/additives, base, and light source. The synthesized diselenide also catalyzes the reduction of hydrogen peroxide into water by following
-
Concise synthesis of a new triterpenoid saponin from the roots of Gypsophila oldhamiana and its derivatives as α-glucosidase inhibitors作者:Qingchao Liu、Tiantian Guo、Fahui Li、Dong LiDOI:10.1039/c6nj01602b日期:——The first synthesis of the triterpenoid saponin 1 and its derivatives 2–3 was efficiently achieved in an efficient and practical strategy with an odourless 2-methyl-5-tert-butylphenyl (Mbp) thioglycoside and a N-phenyltrifluoroacetimidate donor as a key step, and their inhibitory activities against α-glucosidase and α-amylase were evaluated in vitro. The preliminary structure–activity relationship以无味的2-甲基-5-叔丁基苯基(Mbp)硫代糖苷和N-苯基三氟乙酰亚氨酸酯供体为关键步骤,有效而有效地实现了三萜皂苷1及其衍生物2-3的首次合成,和它们对α葡糖苷酶和α淀粉酶抑制活性进行评价体外。初步的结构-活性关系研究表明,C4-CHO和C4-CH 2 OH对α-葡萄糖苷酶抑制活性不是必需的。在这三种化合物中,化合物3表现出对α-葡萄糖苷酶的显着抑制活性,IC 50为值为9.17μM。根据Dixon图上的截距确定为9.35μM的K i,抗α-葡萄糖苷酶的更强皂苷3可能是非竞争性抑制模式。同时,计算化合物1-3的亲脂性,作为药理效力的预测。根据预测的log P值,亲脂性可能与评估的生物学潜能相关。
-
Rapid and efficient conversion of sialyl thioglycosides to sialyl esters via NIS/BF 3 OEt 2 -promoted glycosylation作者:Jiazhe Wang、Rongkun Liu、You YangDOI:10.1016/j.tetlet.2017.05.011日期:2017.6A rapid and efficient one-step conversion of sialyl thioglycosides to sialyl esters was disclosed. Under the promotion of NIS and BF3OEt2, the glycosylation of per-acetylated sialyl thioglycoside with a set of carboxylic acids provided β-sialyl esters as the major products in good to excellent yields within 5 min. Compared with the long-chain alkyl-, aryl- and α,β-unsaturated acids, complete β-selectivities
-
Concise synthesis of two natural steroidal glycosides isolated from Allium schoenoprasum作者:Qingchao Liu、Tiantian Guo、Dong Li、Wenhong LiDOI:10.1007/s11164-015-2106-2日期:2016.3Two natural steroidal glycosides, diosgenin 3-O-α-l-rhamnopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→4)]-β-d-glucopyranoside (1) and laxogenin 3-O-α-l-rhamnopyranosyl-(1→2)-[β-d-glucopyranosyl-(1→4)]-β-d-glucopyranoside (2) with important cytotoxic activity against the HCT 116 and HT-29 human colon cancer cell lines have been efficiently synthesized via straightforward sequential glycosylation reaction with the combined use of N-phenyltrifluoroacetimidates and trichloroacetimidates donors at room temperature. All structures of the synthesized new compounds were identified by 1H NMR, 13C NMR and HRMS spectra.
-
Pseudoenantiomeric glycoclusters: synthesis and testing of heterobivalency in carbohydrate–protein interactions作者:Jasna Brekalo、Guillaume Despras、Thisbe K. LindhorstDOI:10.1039/c9ob00124g日期:——ligands to obtain a library of pseudoenantiomeric glycoclusters. They all have an α-d-mannosyl residue in common as a specific ligand for lectins FimH and ConA, while they differ in the second carbohydrate portion, consisting of a β-d-glucosyl, a β-d-galactosyl or a β-d-glucosaminyl residue as unspecific ligands. The synthesised heteroclusters were tested in standard binding-inhibition assays investigating多价碳水化合物与蛋白质的相互作用是细胞识别过程中的关键事件,并且已经通过合成糖模拟物进行了广泛研究。迄今为止,在多价结构的设计中经常考虑化合价,即与多价支架连接的配体的多重性,但是这些研究并未在分子水平上导致对聚糖识别的结论性理解。在这项工作中,我们通过设计第一个杂二价非对映异构糖簇为碳水化合物-凝集素识别研究增加了一个新的方面,以便研究杂多价和相对配体取向的影响。将分别衍生自d-丝氨酸和l-丝氨酸的两个对映体支架用两个不同的碳水化合物配体糖基化以获得假对映体糖簇库。它们都具有作为凝集素FimH和ConA特异性配体的α-d-甘露糖基残基,而它们在第二个碳水化合物部分(由β-d-葡萄糖基,β-d-半乳糖基或β- d-氨基葡萄糖残基为非特异性配体。在研究FimH介导的细菌粘附和ConA与甘露糖基化表面结合的标准结合抑制试验中测试了合成的异源团簇。观察到两种含假对映异构体葡萄糖的糖簇作为FimH
表征谱图
-
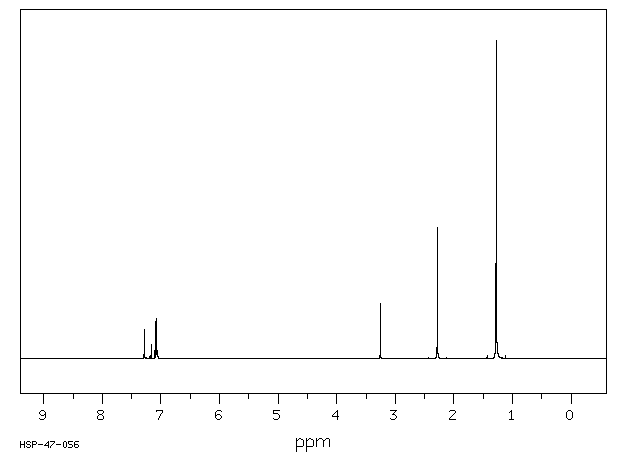
氢谱1HNMR
-
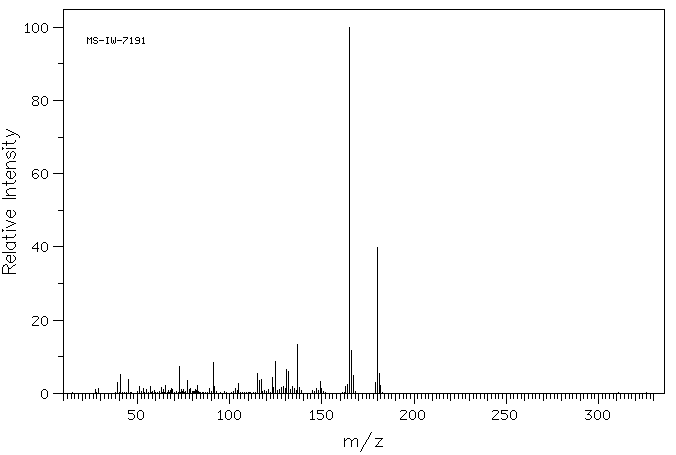
质谱MS
-
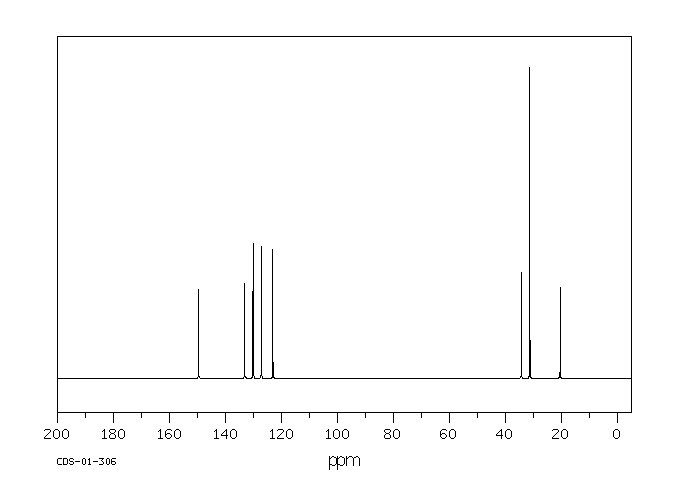
碳谱13CNMR
-
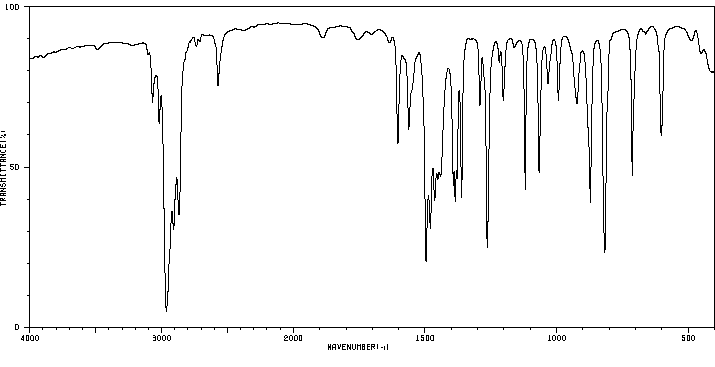
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(βS)-β-氨基-4-(4-羟基苯氧基)-3,5-二碘苯甲丙醇
(S,S)-邻甲苯基-DIPAMP
(S)-(-)-7'-〔4(S)-(苄基)恶唑-2-基]-7-二(3,5-二-叔丁基苯基)膦基-2,2',3,3'-四氢-1,1-螺二氢茚
(S)-盐酸沙丁胺醇
(S)-3-(叔丁基)-4-(2,6-二甲氧基苯基)-2,3-二氢苯并[d][1,3]氧磷杂环戊二烯
(S)-2,2'-双[双(3,5-三氟甲基苯基)膦基]-4,4',6,6'-四甲氧基联苯
(S)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(R)富马酸托特罗定
(R)-(-)-盐酸尼古地平
(R)-(-)-4,12-双(二苯基膦基)[2.2]对环芳烷(1,5环辛二烯)铑(I)四氟硼酸盐
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[((6-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(4-叔丁基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-7-双(3,5-二叔丁基苯基)膦基7''-[(3-甲基吡啶-2-基甲基)氨基]-2,2'',3,3''-四氢-1,1''-螺双茚满
(R)-(+)-4,7-双(3,5-二-叔丁基苯基)膦基-7“-[(吡啶-2-基甲基)氨基]-2,2”,3,3'-四氢1,1'-螺二茚满
(R)-3-(叔丁基)-4-(2,6-二苯氧基苯基)-2,3-二氢苯并[d][1,3]氧杂磷杂环戊烯
(R)-2-[((二苯基膦基)甲基]吡咯烷
(R)-1-[3,5-双(三氟甲基)苯基]-3-[1-(二甲基氨基)-3-甲基丁烷-2-基]硫脲
(N-(4-甲氧基苯基)-N-甲基-3-(1-哌啶基)丙-2-烯酰胺)
(5-溴-2-羟基苯基)-4-氯苯甲酮
(5-溴-2-氯苯基)(4-羟基苯基)甲酮
(5-氧代-3-苯基-2,5-二氢-1,2,3,4-oxatriazol-3-鎓)
(4S,5R)-4-甲基-5-苯基-1,2,3-氧代噻唑烷-2,2-二氧化物-3-羧酸叔丁酯
(4S,4''S)-2,2''-亚环戊基双[4,5-二氢-4-(苯甲基)恶唑]
(4-溴苯基)-[2-氟-4-[6-[甲基(丙-2-烯基)氨基]己氧基]苯基]甲酮
(4-丁氧基苯甲基)三苯基溴化磷
(3aR,8aR)-(-)-4,4,8,8-四(3,5-二甲基苯基)四氢-2,2-二甲基-6-苯基-1,3-二氧戊环[4,5-e]二恶唑磷
(3aR,6aS)-5-氧代六氢环戊基[c]吡咯-2(1H)-羧酸酯
(2Z)-3-[[(4-氯苯基)氨基]-2-氰基丙烯酸乙酯
(2S,3S,5S)-5-(叔丁氧基甲酰氨基)-2-(N-5-噻唑基-甲氧羰基)氨基-1,6-二苯基-3-羟基己烷
(2S,2''S,3S,3''S)-3,3''-二叔丁基-4,4''-双(2,6-二甲氧基苯基)-2,2'',3,3''-四氢-2,2''-联苯并[d][1,3]氧杂磷杂戊环
(2S)-(-)-2-{[[[[3,5-双(氟代甲基)苯基]氨基]硫代甲基]氨基}-N-(二苯基甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[((1S,2S)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2S)-2-[[[[[[((1R,2R)-2-氨基环己基]氨基]硫代甲基]氨基]-N-(二苯甲基)-N,3,3-三甲基丁酰胺
(2-硝基苯基)磷酸三酰胺
(2,6-二氯苯基)乙酰氯
(2,3-二甲氧基-5-甲基苯基)硼酸
(1S,2S,3S,5S)-5-叠氮基-3-(苯基甲氧基)-2-[(苯基甲氧基)甲基]环戊醇
(1S,2S,3R,5R)-2-(苄氧基)甲基-6-氧杂双环[3.1.0]己-3-醇
(1-(4-氟苯基)环丙基)甲胺盐酸盐
(1-(3-溴苯基)环丁基)甲胺盐酸盐
(1-(2-氯苯基)环丁基)甲胺盐酸盐
(1-(2-氟苯基)环丙基)甲胺盐酸盐
(1-(2,6-二氟苯基)环丙基)甲胺盐酸盐
(-)-去甲基西布曲明
龙蒿油
龙胆酸钠
龙胆酸叔丁酯
龙胆酸
龙胆紫-d6
龙胆紫