八甲基环四硅氮烷 | 1020-84-4
中文名称
八甲基环四硅氮烷
中文别名
1,1,3,3,5,5,7,7-八甲基环四硅氮烷
英文名称
2,2,4,4,6,6,8,8-octamethyl-cyclotetrasilazane
英文别名
2,2,4,4,6,6,8,8-octamethyl-cyclo-tetrasilazane;2,2,4,4,6,6,8,8-octamethylcyclotetrasilazane;octamethylcyclotetrasilazane;2,2,4,4,6,6,8,8-Octamethyl-cyclotetrasilazan;2,2,4,4,6,6,8,8-octamethyl-1,3,5,7,2,4,6,8-tetrazatetrasilocane
CAS
1020-84-4
化学式
C8H28N4Si4
mdl
MFCD00046123
分子量
292.679
InChiKey
FIADVASZMLCQIF-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:96-99 °C(lit.)
-
沸点:225 °C756 mm Hg(lit.)
-
密度:0.95
-
闪点:66 °C
-
溶解度:溶于丙酮
-
稳定性/保质期:
在常温常压下保持稳定,应避免与水分直接接触。
计算性质
-
辛醇/水分配系数(LogP):-0.5
-
重原子数:16
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:48.1
-
氢给体数:4
-
氢受体数:4
安全信息
-
TSCA:Yes
-
危险等级:8
-
危险品标志:T
-
安全说明:S45
-
危险类别码:R25
-
WGK Germany:3
-
危险品运输编号:UN 2811 6.1/PG 3
-
RTECS号:GZ4342500
-
海关编码:2915900090
-
包装等级:III
-
危险类别:8
-
储存条件:请将容器密封保存,并储存在阴凉、干燥的地方。
SDS
八甲基环四硅氮烷 修改号码:5
模块 1. 化学品
产品名称: Octamethylcyclotetrasilazane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽会中毒。
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 切勿吸入。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
八甲基环四硅氮烷 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 八甲基环四硅氮烷
百分比: >95.0%(T)
CAS编码: 1020-84-4
俗名: Dimethylsilazane Cyclic Tetramer
分子式: C8H28N4Si4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
八甲基环四硅氮烷 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 氨味
pH: 无数据资料
熔点: 98°C
沸点/沸程 225 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强酸
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氧化硅
模块 11. 毒理学信息
急性毒性: orl-rat LD50:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: GZ4342500
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
八甲基环四硅氮烷 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
副危险性: 第6.1类:毒害品
UN编号: 2923
正式运输名称: 腐蚀性固体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: Octamethylcyclotetrasilazane
修改号码: 5
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第3级
皮肤腐蚀/刺激 1C类
严重损伤/刺激眼睛 第1级
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 危险
危险描述 吞咽会中毒。
造成严重的皮肤灼伤和眼损伤
防范说明
[预防] 切勿吸入。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。
食入:立即呼叫解毒中心/医生。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
皮肤接触:立即去除/脱掉所有被污染的衣物。用水清洗皮肤/淋浴。
被污染的衣物清洗后方可重新使用。
立即呼叫解毒中心/医生。
[储存] 存放处须加锁。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
八甲基环四硅氮烷 修改号码:5
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 八甲基环四硅氮烷
百分比: >95.0%(T)
CAS编码: 1020-84-4
俗名: Dimethylsilazane Cyclic Tetramer
分子式: C8H28N4Si4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
立即呼叫解毒中心/医生。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
立即呼叫解毒中心/医生。
食入: 立即呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用特殊的个人防护用品(针对有毒颗粒的P3过滤式空气呼吸器)。远离溢出物/泄露
紧急措施: 处并处在上风处。
泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果可能,使用封闭系统。如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
存放处须加锁。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统。同时安装淋浴器和洗眼器。
个人防护用品
呼吸系统防护: 防尘面具,自携式呼吸器(SCBA),供气呼吸器等。使用通过政府标准的呼吸器。依
据当地和政府法规。
八甲基环四硅氮烷 修改号码:5
模块 8. 接触控制和个体防护
手部防护: 防渗手套。
眼睛防护: 护目镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防渗防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
固体
外形(20°C):
外观: 晶体-粉末
颜色: 白色类白色
气味: 氨味
pH: 无数据资料
熔点: 98°C
沸点/沸程 225 °C
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 丙酮
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂, 强酸
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx), 氧化硅
模块 11. 毒理学信息
急性毒性: orl-rat LD50:100 mg/kg
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
RTECS 号码: GZ4342500
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
八甲基环四硅氮烷 修改号码:5
模块 12. 生态学信息
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 第8类 腐蚀品
副危险性: 第6.1类:毒害品
UN编号: 2923
正式运输名称: 腐蚀性固体, 有毒的, 不另作详细说明
包装等级: III
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
上下游信息
反应信息
-
作为反应物:描述:参考文献:名称:�ber die gezielte Spaltung von Cyclosilazanen mit Alkoholen und mit Chlorwasserstoff摘要:DOI:10.1007/bf00908794
-
作为产物:参考文献:名称:烷基烷基氮烷和一些相关化合物。摘要:DOI:10.1021/ja01191a106
-
作为试剂:参考文献:名称:Vorbrueggen, Helmut; Krolikiewicz, Konrad, Chemische Berichte, 1984, vol. 117, # 4, p. 1523 - 1541摘要:DOI:
文献信息
-
Beiträge zur chemie der SiN-Bindung, I Darstellung und Zerfall cyclischer N-silylierter Silazane作者:Walter FinkDOI:10.1002/hlca.19620450402日期:——Hexamethylcyclotrisilazan und Octamethylcyclotetrasilazan ergeben mit Butyllithium N-Lithiumcyclosilazane, die mit Trimethylchlorsilan umgesetzt N-silylierte Cyclosilazane liefern. Diese sind z. T. nicht stabil; sie zeigen Zerfallsreaktionen sowie inter- und intramolekulare Silylwanderungen; die stabilen Endprodukte sind zweifach N-silylierte cyclische Di-, Tri- und Tetrasilazane.
-
一种六甲基环三硅氮烷的制备方法申请人:张家港市国泰华荣化工新材料有限公司公开号:CN107778327A公开(公告)日:2018-03-09
-
Process for preparing hexamethylcyclotrisilazane申请人:Shin-Etsu Chemical Co., Ltd.公开号:US05466847A1公开(公告)日:1995-11-14A process for preparing hexamethylcyclo-trisilazane by heating octamethylcyclotetra-silazane in the presence of a catalyst such as a Lewis acid or a sulfur compound of the following formula ##STR1## wherein M represents Ca, Mg, Al, Fe or NH.sub.4, R represents OH, a phenyl group or a substituted phenyl group, x is 0, 1 or 2 and y is 0, 1, 2 or 3 provided that x and y are not zero at the same time, and z is 0, 1, 2 or 3.
-
Method for preparing hexamethyl cyclotrisilazane申请人:Shin-Etsu Chemical Co., Ltd.公开号:US05075474A1公开(公告)日:1991-12-24According to the method for preparing hexamethyl cyclotrisilazane of the present invention, hexamethyl cyclotrisilazane can be obtained by heating a linear or cyclic silazane compound represented by the following general formula: --(Me.sub.2 SiNH).sub.n -- (wherein Me represents a methyl group and n is an integer of not less than 4) in the presence of at least one catalytic compound selected from the group consisting of ammonium salts of arylsulfonic acids and/or aminoarylsulfonic acids and the resulting hexamethyl cyclotrisilazane represented by the formula: --Me.sub.2 SiNH).sub.3 -- can be recovered by distilling off the same outside the reaction system. According to the method of the present invention, highly pure hexamethyl cyclotrisilazane can be industrially prepared in good efficiency and in a high yield. In particular, if octamethyl cyclotetrasilazane which can be industrially prepared from cheap dimethyldichlorosilane is used as a starting material, hexamethyl cyclotrisilazane can also be prepared at a low cost. Thus, the method of the present invention has enough practical value in the organic silicon industries.根据本发明制备六甲基环三硅氮烷的方法,可以通过在至少选择自芳基磺酸铵盐和/或氨基芳基磺酸盐组成的催化化合物存在下,加热下列通式表示的线性或环状硅氮烷化合物:--(Me.sub.2 SiNH).sub.n--(其中Me代表甲基,n为不小于4的整数),从而获得六甲基环三硅氮烷,其表示为以下通式:--Me.sub.2 SiNH).sub.3--,可以通过在反应体系外蒸馏得到。根据本发明的方法,可以高效率、高产率地工业制备高纯度的六甲基环三硅氮烷。特别是,如果以可以从廉价的二甲基二氯硅烷工业制备的八甲基环四硅氮烷作为起始材料,则六甲基环三硅氮烷也可以以低成本制备。因此,本发明的方法在有机硅行业具有足够的实用价值。
-
Experimental Charge Density Studies of Cyclotetrasilazane and Metal Complexes Containing the Di- and Tetraanion作者:Nikolaus Kocher、Carola Selinka、Dirk Leusser、Daniel Kost、Inna Kalikhman、Dietmar StalkeDOI:10.1002/zaac.200400277日期:2004.10Si-N(M), Si-N(H, M) and M-N bonds and serve as bench-mark systems to study polar bonds by high-resolution low-temperature X-ray structure analysis. Experimental charge density studies reveal highly polar Si-N bonds with remarkable ionic contribution, even in the non-metallated starting material 1. The Li-N and Li-O bonds have to be classified as almost purely ionic bonds with topological properties not母体八甲基环四硅氮烷(c-NH-SiMe2-)4的同源系列,(1),锂络合物[(THF)2Li2(cN-SiMe2-NH-SiMe2-)2]2,(2),含有环状双阴离子, 和 [(THF)2LiAl(cN-SiMe2-)4]2, (3), 可容纳前所未有的四阴离子 [Me2SiN]4- 以研究在各种金属存在下共价 Si-N 单键的性质. 这些模型化合物显示出多种多样的 Si-N(H)、Si-N(M)、Si-N(H, M) 和 MN 键,可作为基准系统通过高分辨率低温 X 研究极性键射线结构分析。实验电荷密度研究揭示了具有显着离子贡献的高极性 Si-N 键,即使在非金属化起始材料 1 中也是如此。Li-N 和 Li-O 键必须归类为几乎纯离子键,其拓扑特性与 NaCl 确定的拓扑特性相近。环四硅氮烷和二和四阴离子金属配合物的实验电子密度研究 从八甲基环四硅氮烷 (c-NH-SiMe2-)4,
表征谱图
-
氢谱1HNMR
-
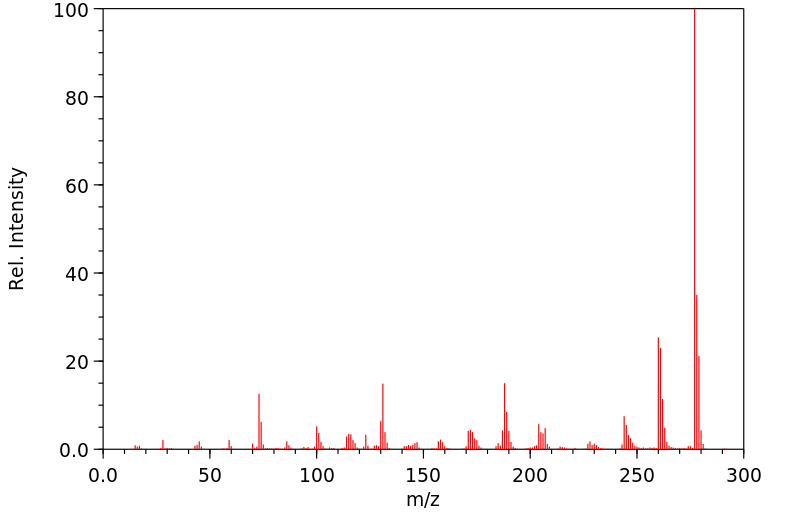
质谱MS
-
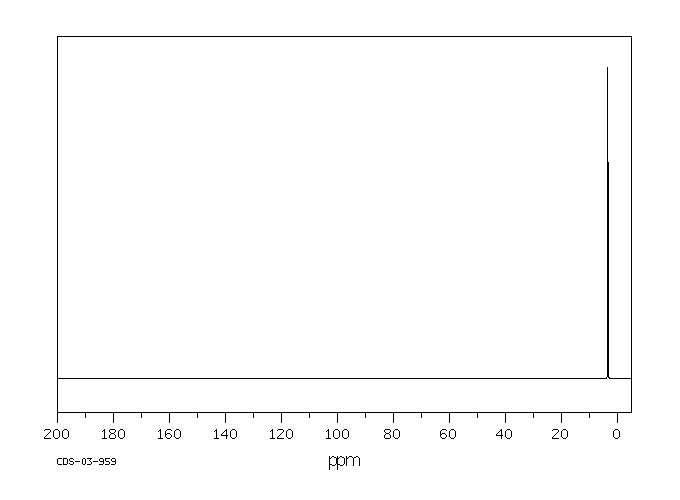
碳谱13CNMR
-
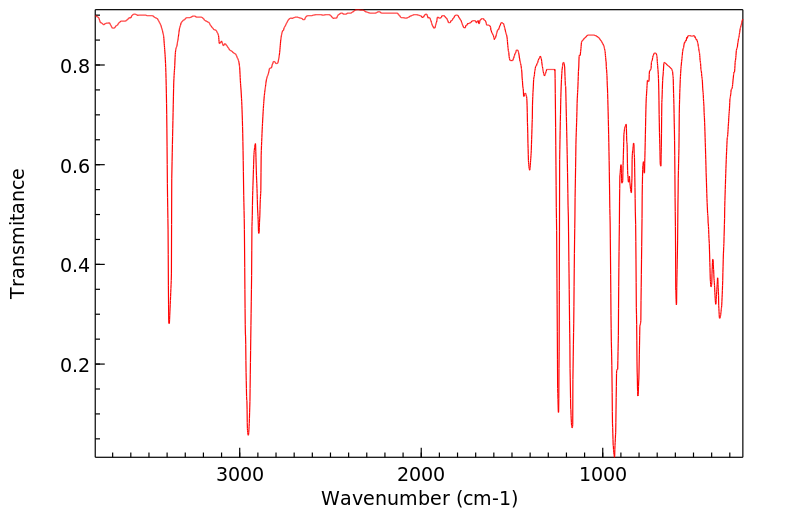
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(2-溴乙氧基)-特丁基二甲基硅烷
鲸蜡基聚二甲基硅氧烷
骨化醇杂质DCP
马沙骨化醇中间体
马来酸双(三甲硅烷)酯
顺式-二氯二(二甲基硒醚)铂(II)
顺-N-(1-(2-乙氧基乙基)-3-甲基-4-哌啶基)-N-苯基苯酰胺
降钙素杂质13
降冰片烯基乙基三甲氧基硅烷
降冰片烯基乙基-POSS
间-氨基苯基三甲氧基硅烷
镓,二(1,1-二甲基乙基)甲基-
镁,氯[[二甲基(1-甲基乙氧基)甲硅烷基]甲基]-
锑,二溴三丁基-
铷,[三(三甲基甲硅烷基)甲基]-
铂(0)-1,3-二乙烯-1,1,3,3-四甲基二硅氧烷
钾(4-{[二甲基(2-甲基-2-丙基)硅烷基]氧基}-1-丁炔-1-基)(三氟)硼酸酯(1-)
金刚烷基乙基三氯硅烷
酰氧基丙基双封头
达格列净杂质
辛醛,8-[[(1,1-二甲基乙基)二甲基甲硅烷基]氧代]-
辛甲基-1,4-二氧杂-2,3,5,6-四硅杂环己烷
辛基铵甲烷砷酸盐
辛基衍生化硅胶(C8)ZORBAX?LP100/40C8
辛基硅三醇
辛基甲基二乙氧基硅烷
辛基三甲氧基硅烷
辛基三氯硅烷
辛基(三苯基)硅烷
辛乙基三硅氧烷
路易氏剂-3
路易氏剂-2
路易士剂
试剂Cyanomethyl[3-(trimethoxysilyl)propyl]trithiocarbonate
试剂3-[Tris(trimethylsiloxy)silyl]propylvinylcarbamate
试剂3-(Trimethoxysilyl)propylvinylcarbamate
试剂2-(Trimethylsilyl)cyclopent-2-en-1-one
试剂11-Azidoundecyltriethoxysilane
西甲硅油杂质14
衣康酸二(三甲基硅基)酯
苯胺,4-[2-(三乙氧基甲硅烷基)乙基]-
苯磺酸,羟基-,盐,单钠聚合甲醛,1,3,5-三嗪-2,4,6-三胺和脲
苯甲醇,a-[(三苯代甲硅烷基)甲基]-
苯并磷杂硅杂英,5,10-二氢-10,10-二甲基-5-苯基-
苯基二甲基氯硅烷
苯基二甲基乙氧基硅
苯基二甲基(2'-甲氧基乙氧基)硅烷
苯基乙酰氧基三甲基硅烷
苯基三辛基硅烷
苯基三甲氧基硅烷