1-甲基-1,2,3,6-四氢吡啶 | 694-55-3
中文名称
1-甲基-1,2,3,6-四氢吡啶
中文别名
——
英文名称
N-methyl-3,4-didehydropiperidine
英文别名
N-methyl-1,2,3,6-tetrahydropyridine;1-methyl-1,2,5,6-tetrahydropyridine;1-methyl-1,2,3,6-tetrahydropyridine;1-methyl-3,6-dihydro-2H-pyridine
CAS
694-55-3
化学式
C6H11N
mdl
——
分子量
97.16
InChiKey
AUFIRGPROMANKW-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:198-202 °C
-
沸点:112.5 °C
-
密度:0.876±0.06 g/cm3(Predicted)
-
保留指数:1030
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):1
-
重原子数:7
-
可旋转键数:0
-
环数:1.0
-
sp3杂化的碳原子比例:0.67
-
拓扑面积:3.2
-
氢给体数:0
-
氢受体数:1
安全信息
-
海关编码:2933399090
-
储存条件:密封储存,应存放在阴凉、干燥的库房中。
SDS
| Name: | 1-Methyl-1 2 3 6-tetrahydropyridine hydro- chloride 98% Material Safety Data Sheet |
| Synonym: | |
| CAS: | 694-55-3 |
Synonym:
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 694-55-3 | 1-methyl-1,2,3,6-tetrahydropyridine hy | 98 | 211-773-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
Dust may cause mechanical irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause digestive tract disturbances. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid immediately.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
If victim is conscious and alert, give 2-4 cupfuls of milk or water.
Never give anything by mouth to an unconscious person. Get medical aid immediately.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up or absorb material, then place into a suitable clean, dry, closed container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed.
Avoid ingestion and inhalation. Use with adequate ventilation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 694-55-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: off-white
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 198.00 - 200.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C6H11N.HCl
Molecular Weight: 133.62
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, strong oxidants.
Incompatibilities with Other Materials:
Strong oxidizing agents.
Hazardous Decomposition Products:
Irritating and toxic fumes and gases.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 694-55-3 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
1-methyl-1,2,3,6-tetrahydropyridine hydro- chloride, 98% - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 694-55-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 694-55-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 694-55-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 1,2,3,6-四氢吡啶 1,2,3,6-tetrahydropyridine 694-05-3 C5H9N 83.1332
反应信息
-
作为反应物:描述:参考文献:名称:Lukes; Pliml, Collection of Czechoslovak Chemical Communications, 1954, vol. 19, p. 502摘要:DOI:
-
作为产物:描述:参考文献:名称:Exploratory and mechanistic aspects of the electron-transfer photochemistry of olefin-N-heteroaromatic cation systems摘要:DOI:10.1021/ja00343a022
文献信息
-
Some reactions of 1-methyl-1,2,3,4-tetrahydropyridine with organic azides. Synthesis of 1-methylpiperidylidene-2-sulfon(cyan)amides作者:B. K. Warren、E. E. KnausDOI:10.1002/jhet.5570190562日期:1982.9The 1,3-dipolarcycloaddition reaction of 1-methyl-1,2,3,4-tetrahydropyridine (1) with organic azides 2 affords 1-methylpiperidylidene-2-sulfon(cyan)amides 4 in high yield. The reaction proceeds via a 2,3,4,9-tetrazabicyclo[4.3.0]non-3-ene intermediate. Acid hydrolysis of 4d gives rise to 1-methyl-2-piperidone.
-
[EN] PYRROLOTRIAZINE COMPOUNDS AS KINASE INHIBITORS<br/>[FR] COMPOSES DE PYRROLOTRIAZINE SERVANT D'INHIBITEURS DE KINASES申请人:BRISTOL MYERS SQUIBB CO公开号:WO2005066176A1公开(公告)日:2005-07-21The present invention provides compounds of formula (I); and pharmaceutically acceptable salts thereof. The formula (I) compounds inhibit tyrosine kinase activity of growth factor receptors such as HER1, HER2 and HER4 thereby making them useful as antiproliferative agents. The formula (I) compounds are also useful for the treatment of other diseases associated with signal transduction pathways operating through growth factor receptors.
-
Base catalysed rearrangements involving ylide intermediates. Part 7. The rearrangements of allyl(pentadienyl)- and propynyl(pentadienyl)ammonium cations. The [5,4] sigmatropic rearrangement作者:Trevor Laird、W. David Ollis、Ian O. SutherlandDOI:10.1039/p19800002033日期:——The base catalysed rearrangements of the cations (7), (17), (22), and (27) gave the enamines (9), (18), (23), and (28), which on hydrolysis yielded the aldehydes (10), (19), (24), and (29) respectively. The reactions are shown to be concerted [5,4] sigmatropic rearrangements proceeding via a nine-membered transition state involving 10π electrons. The base catalysed rearrangements of the 3-phenylprop-2-ynyl
-
Catalytic Asymmetric Hydrogen Migration of Allylamines作者:Sei Otsuka、Kazuhide TaniDOI:10.1055/s-1991-26541日期:——This review describes some preparative aspects and practical applications of BINAP-Rh(I) catalyzed enantioselective isomerization of prochiral allylamines recently developed through the joint effort of several groups in Japan. In addition, the novel, sophisticated reaction mechanisms are discussed. 1. Introduction 2. Catalyst Development 2.1. Cobalt Catalysts 2.2. Rhodium Catalysts 2.3. The Ligand BINAP 3. Preparative Aspects 3.1. Isomerization on a Laboratory Scale 3.2. The Industrial Process 4. Substrates and the Scope 4.1. Allylamine 4.2. Allyl Alcohol 5. Reaction Mechanisms 5.1. Catalytic Pathways 5.2. Mechanism of Chiral Recognition 5.3. Conclusion 6. Applications 6.1. (-)-Menthol 6.2. Citronellol 6.3. Citronellal Derivatives 6.4. α-Tocopherol Side Chain 6.5. Methoprene
-
A convenient racemic synthesis of two isomeric tetrahydropyridyl alkaloids: Isoanatabine and anatabine作者:Anne Rouchaud、William R. KemDOI:10.1002/jhet.359日期:——contributed to the knowledge of the mechanism of this oxidative rearrangement. On the other hand, the reduction of 1‐methylpyridinium iodide with sodium borohydride and with potassium cyanide present since the start of the reaction in a two layer ether‐water system, gave 2‐cyano‐1‐methyl‐4‐piperideine. This was transformed into (±)‐anatabine by the same sequence of reactions used for the synthesis of (±)‐isoanatabineAnatabine是烟草的主要生物碱,其异构体isoanatabine最近在海洋蠕虫中被发现。用硼氢化钠还原1-甲基碘化碘,得到1-甲基-3-哌啶,然后用过氧化氢转化成N-氧化物。N氧化物先后与三氟乙酸酐和氰化钾反应,生成2氰基1甲基3哌啶。它与3-吡啶基氯化镁反应生成(±)-N-甲基-异他他滨。这与转化的米氯过苯甲酸入Ñ氧化物这是Ñ用硫酸铁(II)脱甲基,得到(±)-异anatabatin。通过N氧化物分解进行N脱甲基化的文献方法的连续应用,有助于人们了解这种氧化重排的机理。另一方面,自两层醚-水系统中反应开始以来,用硼氢化钠和氰化钾还原1-甲基吡啶碘化物,得到2-氰基-1-甲基-4-哌啶。通过合成(±)-异anatabine的相同反应顺序,将其转化为(±)-anatabine。J.杂环化学。(2010)。
表征谱图
-
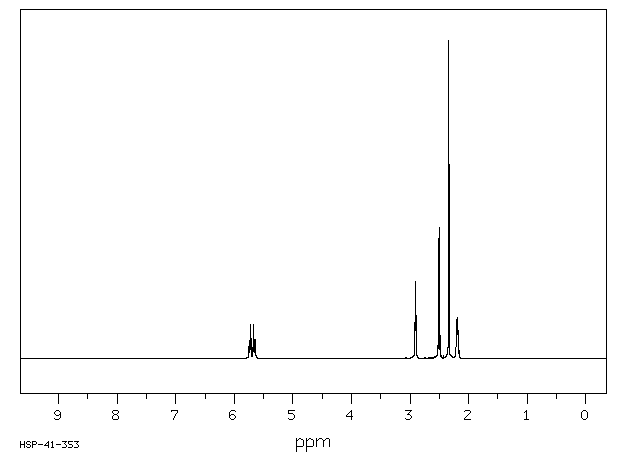
氢谱1HNMR
-
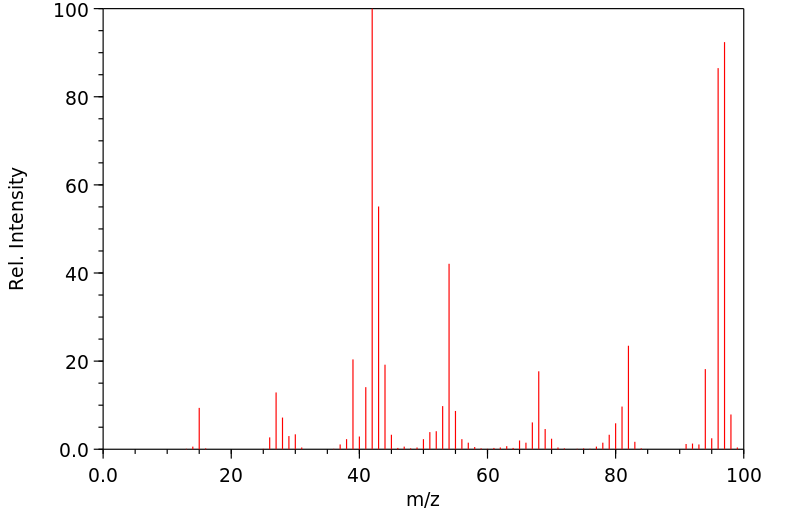
质谱MS
-
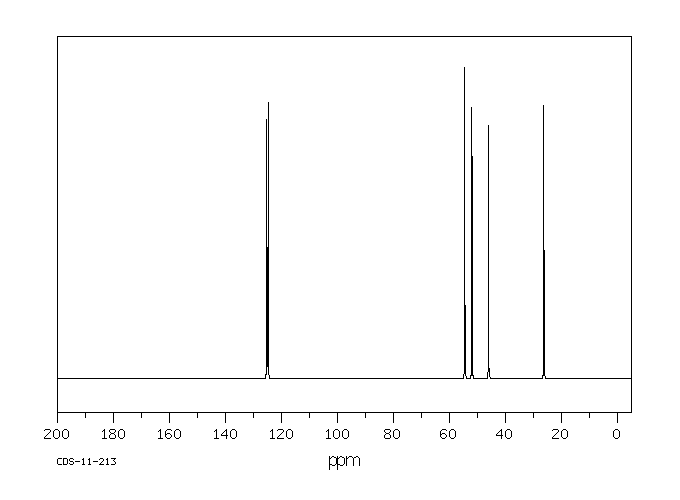
碳谱13CNMR
-
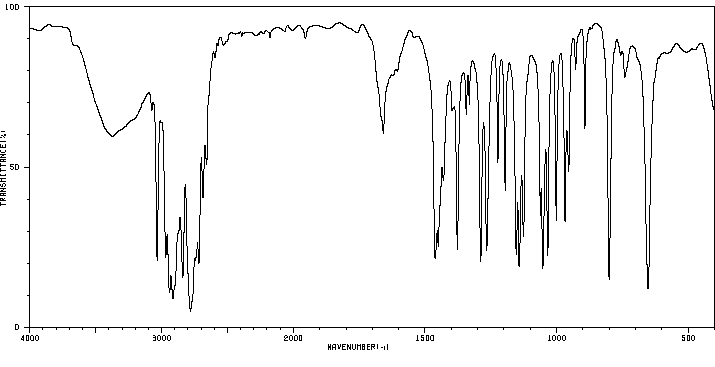
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-