4,4'-二氨基-2,2'-联吡啶 | 18511-69-8
中文名称
4,4'-二氨基-2,2'-联吡啶
中文别名
——
英文名称
4,4'-diamino-2,2'-bipyridyl
英文别名
4,4'-diamino-2,2'-bipyridine;4,4′-diamino-2,2′-bipyridine;2,2'-bipyridine-4,4'-diamine;4,4’-diamino-2,2’-bipyridine;dabpy;[2,2′-bipyridine]-4,4′-diamine;[2,2'-Bipyridine]-4,4'-diamine;2-(4-aminopyridin-2-yl)pyridin-4-amine
CAS
18511-69-8
化学式
C10H10N4
mdl
MFCD08704188
分子量
186.216
InChiKey
WTHJTVKLMSJXEV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:>270°C (dec.)
-
沸点:521.0±50.0 °C(Predicted)
-
密度:1.277±0.06 g/cm3(Predicted)
-
溶解度:可溶于DMSO(少许)、甲醇(少许)
计算性质
-
辛醇/水分配系数(LogP):0.1
-
重原子数:14
-
可旋转键数:1
-
环数:2.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:77.8
-
氢给体数:2
-
氢受体数:4
安全信息
-
危险性防范说明:P305+P351+P338
-
危险性描述:H315,H319
-
储存条件:室温
SDS
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 1. 化学品
产品名称: 4,4'-Diamino-2,2'-bipyridyl
修改号码: 5.3
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二氨基-2,2'-联吡啶
百分比: >98.0%(GC)(T)
CAS编码: 18511-69-8
俗名: 4,4'-Diamino-2,2'-bipyridine
分子式: C10H10N4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 7. 操作处置与储存
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 热甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
模块 1. 化学品
产品名称: 4,4'-Diamino-2,2'-bipyridyl
修改号码: 5.3
模块 2. 危险性概述
GHS分类
物理性危害 未分类
健康危害
急性毒性(经口) 第4级
急性毒性(经皮) 第4级
急性毒性(吸入) 第4级
皮肤腐蚀/刺激 第2级
严重损伤/刺激眼睛 2A类
环境危害 未分类
GHS标签元素
图标或危害标志
信号词 警告
危险描述 吸入或皮肤接触或吞咽有害。
造成皮肤刺激
造成严重眼刺激
防范说明
[预防] 避免吸入。
只能在室外或通风良好的环境下使用。
使用本产品时切勿吃东西,喝水或吸烟。
处理后要彻底清洗双手。
穿戴防护手套/护目镜/防护面具。
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 2. 危险性概述
[急救措施] 吸入:将受害者移到新鲜空气处,在呼吸舒适的地方保持休息。若感不适,呼叫解毒
中心/医生。
食入:若感不适,呼叫解毒中心/医生。漱口。
眼睛接触:用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续冲洗。
眼睛接触:求医/就诊
皮肤接触:用大量肥皂和水轻轻洗。
若皮肤刺激:求医/就诊。
被污染的衣物清洗后方可重新使用。
若感不适:呼叫解毒中心/医生。
[废弃处置] 根据当地政府规定把物品/容器交与工业废弃处理机构。
模块 3. 成分/组成信息
单一物质/混和物 单一物质
化学名(中文名): 4,4'-二氨基-2,2'-联吡啶
百分比: >98.0%(GC)(T)
CAS编码: 18511-69-8
俗名: 4,4'-Diamino-2,2'-bipyridine
分子式: C10H10N4
模块 4. 急救措施
吸入: 将受害者移到新鲜空气处,保持呼吸通畅,休息。若感不适立即呼叫解毒中心/医生。
皮肤接触: 立即去除/脱掉所有被污染的衣物。用大量肥皂和水轻轻洗。
若皮肤刺激或发生皮疹:求医/就诊。
眼睛接触: 用水小心清洗几分钟。如果方便,易操作,摘除隐形眼镜。继续清洗。
如果眼睛刺激:求医/就诊。
食入: 若感不适,呼叫解毒中心/医生。漱口。
紧急救助者的防护: 救援者需要穿戴个人防护用品,比如橡胶手套和气密性护目镜。
模块 5. 消防措施
合适的灭火剂: 干粉,泡沫,雾状水,二氧化碳
特殊危险性: 小心,燃烧或高温下可能分解产生毒烟。
特定方法: 从上风处灭火,根据周围环境选择合适的灭火方法。
非相关人员应该撤离至安全地方。
周围一旦着火:如果安全,移去可移动容器。
消防员的特殊防护用具: 灭火时,一定要穿戴个人防护用品。
模块 6. 泄漏应急处理
个人防护措施,防护用具, 使用个人防护用品。远离溢出物/泄露处并处在上风处。
紧急措施: 泄露区应该用安全带等圈起来,控制非相关人员进入。
环保措施: 防止进入下水道。
控制和清洗的方法和材料: 清扫收集粉尘,封入密闭容器。注意切勿分散。附着物或收集物应该立即根据合适的
法律法规处置。
模块 7. 操作处置与储存
处理
技术措施: 在通风良好处进行处理。穿戴合适的防护用具。防止粉尘扩散。处理后彻底清洗双手
和脸。
注意事项: 如果粉尘或浮质产生,使用局部排气。
操作处置注意事项: 避免接触皮肤、眼睛和衣物。
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 7. 操作处置与储存
贮存
储存条件: 保持容器密闭。存放于凉爽、阴暗处。
远离不相容的材料比如氧化剂存放。
包装材料: 依据法律。
模块 8. 接触控制和个体防护
工程控制: 尽可能安装封闭体系或局部排风系统,操作人员切勿直接接触。同时安装淋浴器和洗
眼器。
个人防护用品
呼吸系统防护: 防尘面具。依据当地和政府法规。
手部防护: 防护手套。
眼睛防护: 安全防护镜。如果情况需要,佩戴面具。
皮肤和身体防护: 防护服。如果情况需要,穿戴防护靴。
模块 9. 理化特性
外形(20°C): 固体
外观: 晶体-粉末
颜色: 白色-微浅黄色
气味: 无资料
pH: 无数据资料
熔点: 无资料
沸点/沸程 无资料
闪点: 无资料
爆炸特性
爆炸下限: 无资料
爆炸上限: 无资料
密度: 无资料
溶解度:
[水] 无资料
[其他溶剂]
溶于: 热甲醇
模块 10. 稳定性和反应性
化学稳定性: 一般情况下稳定。
危险反应的可能性: 未报道特殊反应性。
须避免接触的物质 氧化剂
危险的分解产物: 一氧化碳, 二氧化碳, 氮氧化物 (NOx)
模块 11. 毒理学信息
急性毒性: 无资料
对皮肤腐蚀或刺激: 无资料
对眼睛严重损害或刺激: 无资料
生殖细胞变异原性: 无资料
致癌性:
IARC = 无资料
NTP = 无资料
生殖毒性: 无资料
4,4'-二氨基-2,2'-联吡啶 修改号码:5.3
模块 12. 生态学信息
生态毒性:
鱼类: 无资料
甲壳类: 无资料
藻类: 无资料
残留性 / 降解性: 无资料
潜在生物累积 (BCF): 无资料
土壤中移动性
log水分配系数: 无资料
土壤吸收系数 (Koc): 无资料
亨利定律 无资料
constaNT(PaM3/mol):
模块 13. 废弃处置
如果可能,回收处理。请咨询当地管理部门。建议在可燃溶剂中溶解混合,在装有后燃和洗涤装置的化学焚烧炉中
焚烧。废弃处置时请遵守国家、地区和当地的所有法规。
模块 14. 运输信息
联合国分类: 与联合国分类标准不一致
UN编号: 未列明
模块 15. 法规信息
《危险化学品安全管理条例》(2002年1月26日国务院发布,2011年2月16日修订): 针对危险化学品的安全使用、
生产、储存、运输、装卸等方面均作了相应的规定。
模块16 - 其他信息
N/A
制备方法与用途
4,4'-二氨基-2,2'-联吡啶是一种有机杂环化合物,主要用于生化试剂。
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 2,2'-联吡啶 [2,2]bipyridinyl 366-18-7 C10H8N2 156.187 4,4'-二氯-2,2'-联吡啶 4,4'-dichloro-2,2'-bipyridine 1762-41-0 C10H6Cl2N2 225.077 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2-[4-(二甲氨基)吡啶-2-基]-N,N-二甲基吡啶-4-胺 4,4'-bis(dimethylamino)-2,2'-bipyridine 85698-56-2 C14H18N4 242.324 —— 4,4'-diisothiocyanato-2,2'-bipyridine 1289385-91-6 C12H6N4S2 270.338 —— 4,4'-bisacetamido-2,2'-bipyridyl 52597-40-7 C14H14N4O2 270.291 —— 4,4′-bis(di-p-anisylamino)-2,2′-bipyridine —— C38H34N4O4 610.712 4,4'-二氟-2,2'-二吡啶 4,4'-difluoro-2,2'-bipyridine 1189458-67-0 C10H6F2N2 192.168 4,4'-二碘-2,2'-联吡啶 4,4'-diiodo-2,2'-bipyridine 831225-81-1 C10H6I2N2 407.98
反应信息
-
作为反应物:描述:4,4'-二氨基-2,2'-联吡啶 在 pyridine hydrofluoride 、 sodium nitrite 作用下, 以 甲苯 为溶剂, 反应 18.0h, 以36%的产率得到4,4'-二氟-2,2'-二吡啶参考文献:名称:Photoinduced solid-state coloring behavior of boronium complexes摘要:Boronium complexes bearing a 9-borabicyclononane framework with a bipyridine-type ligand display photoinduced solid-state coloring behavior. While the identity of the substituents on the boron atom is critical to gain photoresponsive capability, modifying the nitrogen-containing ligand structure and its substituents provides a wide variation in the photoinduced solid color. (C) 2016 Elsevier Ltd. All rights reserved.DOI:10.1016/j.tetlet.2016.10.094
-
作为产物:描述:2-氯-4-氨基吡啶 在 dibromobis(triphenylphosphine)nickel(II) 、 盐酸羟胺 、 四乙基碘化铵 、 对甲苯磺酸 、 二乙胺 、 lithium chloride 、 锌 作用下, 以 四氢呋喃 、 乙醇 、 水 、 甲苯 为溶剂, 生成 4,4'-二氨基-2,2'-联吡啶参考文献:名称:面向变构受体 - β-环糊精功能化的 2,2'-联吡啶及其金属配合物的合成。摘要:在此,我们提出了三种新的 2,2'-联吡啶,它们以不同的取代模式携带两个 β-环糊精部分。当与锌 (II) 或铜 (I) 离子(或它们的配合物)配位时,这些化合物会发生构象变化并在“开放”和“封闭”形式之间切换,从而将环糊精部分彼此结合或分离。DOI:10.3762/bjoc.10.77
-
作为试剂:描述:参考文献:名称:一种联吡啶类锰催化剂催化氧化胺合成酰胺的方法摘要:本发明公开了一种联吡啶类锰催化剂催化氧化胺合成酰胺的方法,该方法以联吡啶类配体与廉价金属锰配位后形成的联吡啶锰配合物做为催化剂,以清洁、环保的双氧水作为氧化剂,实现了廉价金属锰催化的N邻位sp3 C‑H键的氧化,实现了由胺直接氧化得到酰胺。与现有方法相比,本发明所用催化剂价格低廉,制备方法简单,原料易得,并且有催化剂用量少、底物范围广、反应条件温和、操作简单、绿色环保、反应时间短、产率高、选择性好、工业化成本低等优点。公开号:CN109824465B
文献信息
-
Electronic effects on reactivity and anticancer activity by half-sandwich N,N-chelated iridium(<scp>iii</scp>) complexes作者:Lihua Guo、Hairong Zhang、Meng Tian、Zhenzhen Tian、Yanjian Xu、Yuliang Yang、Hongwei Peng、Peng Liu、Zhe LiuDOI:10.1039/c8nj03360a日期:——The synthesis and characterization of a series of organometallic half-sandwich N,N-chelated iridium(III) complexes bearing a range of electron-donating and withdrawing substituents were described. The X-ray crystal structures of complexes 1, 3 and 5 have been determined. This work demonstrated how the aqueous chemistry, catalytic activity in converting coenzyme NADH to NAD+ and anticancer activity描述了一系列带有一系列给电子和吸电子取代基的有机金属半三明治N,N螯合铱(III)配合物的合成和表征。复合物的X射线晶体结构1,3和5已经被确定。这项工作证明了如何通过修饰配体电子扰动来控制和微调水溶液的化学性质,将辅酶NADH转化为NAD +的催化活性和抗癌活性。通常,在联吡啶环上引入吸电子基团(–Cl和–NO 2)会提高抗癌活性,而给电子基团(–NH 2,-OH和-OCH 3)降低了抗癌活性。带有强吸电子NO 2基团的配合物6表现出最高的抗癌活性(7.3±1.2μM),约为。在A549细胞系中的活性是顺铂的三倍。值得注意的是,复合物1和6观察到对癌细胞的选择性细胞毒性超过正常细胞。DNA结合似乎不是抗癌的主要机制。然而,水化学,细胞凋亡和细胞周期显示出与抗癌活性类似的对配体电子干扰的依赖性,似乎共同促进了这些复合物的抗癌效力。这项工作可能为增强这些N,N螯合的有机金属半夹心铱(III)配合物的抗癌活性提供替代策略。
-
Manganese-Catalyzed Achmatowicz Rearrangement Using Green Oxidant H<sub>2</sub>O<sub>2</sub>作者:Qingzhao Xing、Zhe Hao、Jing Hou、Gaoqiang Li、Ziwei Gao、Jing Gou、Chaoqun Li、Binxun YuDOI:10.1021/acs.joc.1c00858日期:2021.7.16catalytic methods for the oxidative furan-recyclizations remain scarcely investigated. Given this, we report a means of manganese-catalyzed oxidations of furan with low loading, achieving the Achmatowicz rearrangement in the presence of hydrogen peroxide as an environmentally benign oxidant under mild conditions with wide functional group compatibility.
-
Re <sup>I</sup> Tricarbonyl Complexes as Coordinate Covalent Inhibitors for the SARS‐CoV‐2 Main Cysteine Protease作者:Johannes Karges、Mark Kalaj、Milan Gembicky、Seth M. CohenDOI:10.1002/anie.202016768日期:2021.5.3syndrome—coronavirus 2 (SARS‐CoV‐2) has impacted the quality of life and cost hundreds‐of‐thousands of lives worldwide. Based on its global spread and mortality, there is an urgent need for novel treatments which can combat this disease. To date, the 3‐chymotrypsin‐like protease (3CLpro), which is also known as the main protease, is considered among the most important pharmacological targets. The vast majority
-
Design and synthesis of 4,4′-π-conjugated[2,2′]-bipyridines: a versatile class of tunable chromophores and fluorophores作者:Maury、Guegana、Renouard、Hilton、Dupau、Sandon、Toupet、Le BozecDOI:10.1039/b106096c日期:2001.12.3A series of 4,4â²-disubstituted[2,2â²]-bipyridines, featuring Ï-conjugated substituents such as donor-(acceptor-) substituted styryl, thienylvinyl, phenylimino and phenylazo groups, have been synthesized. X-Ray structures are provided for three ligands containing âCCâ, âCNâ and âNNâ linkers, respectively. These chromophores display good to excellent thermal stabilities with decomposition temperatures of up to 350â°C. The strong influence of the nature of the endgroups and Ï-linkers on the optical properties is discussed. The stepwise protonation of 4,4â²-dibutylaminostyryl-[2,2â²]-bipyridine and its coordination behavior to different metallic moieties [Zn(II), Hg(II), Pd(II), Re(I), Re(VII)] have also been investigated. It is found that the absorption and emission maxima can be easily tuned by these exogenous additives over a wide range of wavelengths (360â<âλabsâ<â560 nm ; 482â<âλemâ<â646 nm).合成了一系列4,4'-二取代的[2,2'-]联吡啶,其中的π-共轭取代基包括供体(受体)取代的苯乙烯基、噻吩乙烯基、苯亚胺基和苯偶氮基团。提供了三个含有-CC-、-CN-和-NN-连接子的配体的X射线结构。这些发色团显示了良好的至优良的热稳定性,分解温度高达350°C。讨论了末端基团和π-连接子性质对光学特性的强烈影响。还研究了4,4'-二丁氨基苯乙烯基-[2,2'-]联吡啶的分级质子化和其与不同金属部分[Zn(II)、Hg(II)、Pd(II)、Re(I)、Re(VII)]的配位行为。发现这些外源添加剂可以轻易地在广泛的波长范围内调节吸收和发射最大值(360 < λabs < 560 nm ; 482 < λem < 646 nm)。
-
Controlling Hydrogen Evolution during Photoreduction of CO<sub>2</sub> to Formic Acid Using [Rh(R-bpy)(Cp*)Cl]<sup>+</sup> Catalysts: A Structure–Activity Study作者:Tanya K. Todorova、Tran Ngoc Huan、Xia Wang、Hemlata Agarwala、Marc FontecaveDOI:10.1021/acs.inorgchem.9b00371日期:2019.5.20photochemical reduction of CO2 to formic acid catalyzed by a series of [Rh(4,4′-R-bpy)(Cp*)Cl]+ and [Rh(5,5′-COOH-bpy)(Cp*)Cl]+ complexes (Cp* = pentamethylcyclopentadienyl, bpy = 2,2′-bipyridine, and R = OCH3, CH3, H, COOC2H5, CF3, NH2, or COOH) was studied to assess how modifications in the electronic structure of the catalyst affect its selectivity, defined as the HCOOH:H2 product ratio. A direct molecular-level一系列[Rh(4,4'-R-bpy)(Cp *)Cl] +和[Rh(5,5'-COOH-bpy)(Cp *)催化将CO 2光化学还原为甲酸研究了Cl] +配合物(Cp * =五甲基环戊二烯基,bpy = 2,2'-联吡啶,R = OCH 3,CH 3,H,COOC 2 H 5,CF 3,NH 2或COOH),以评估修饰方式催化剂的电子结构中的H 2 O 3影响其选择性,定义为HCOOH∶H 2产物比。官能团对CO 2初始反应速率的直接分子水平影响对质子还原反应。密度泛函理论计算首次阐明了[RhH]和[Cp * H]互变异构体的各自作用,认识到氢化铑是这两个反应的关键因素。尤其是,我们的计算解释了观察到的给电子取代基通过降低Rh–H键的羟基而倾向于促进CO 2还原的趋势,与带有电子的取代基相比,导致向甲酸生成的氢化物转移势垒更低-抽出的性质更有利于将质子还原为氢。
表征谱图
-
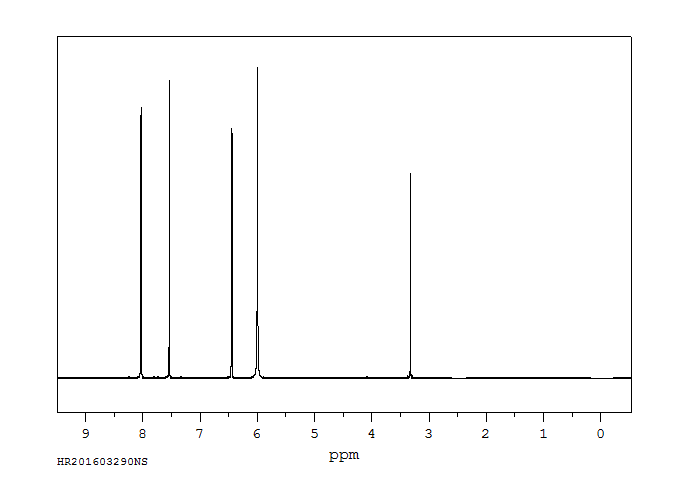
氢谱1HNMR
-
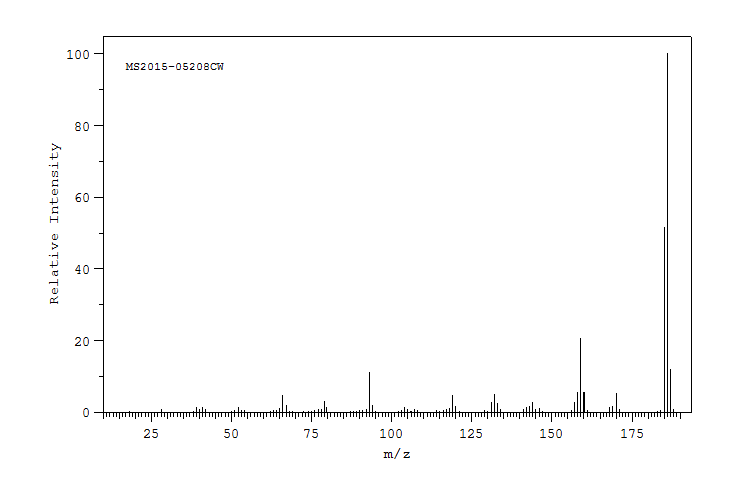
质谱MS
-
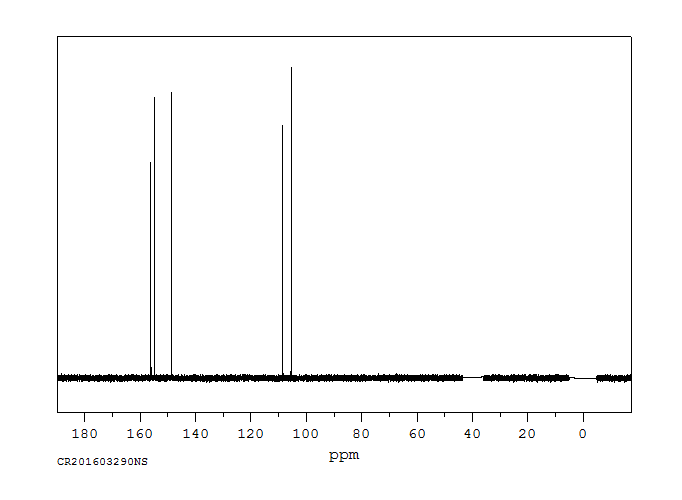
碳谱13CNMR
-
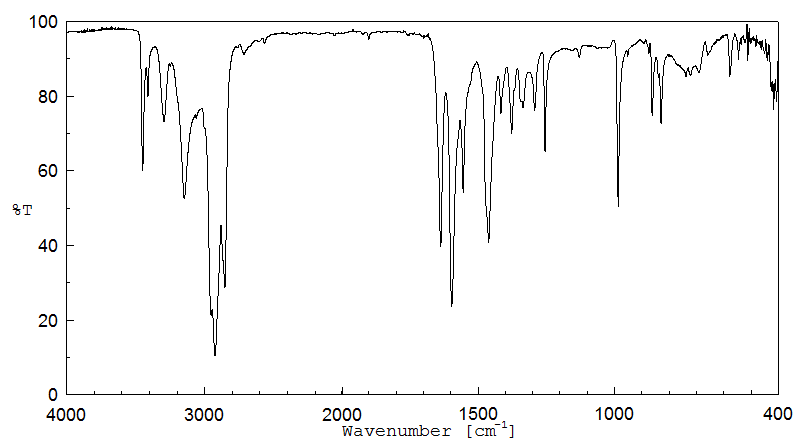
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-氨氯地平-d4
(R,S)-可替宁N-氧化物-甲基-d3
(R)-(+)-2,2'',6,6''-四甲氧基-4,4''-双(二苯基膦基)-3,3''-联吡啶(1,5-环辛二烯)铑(I)四氟硼酸盐
(R)-N'-亚硝基尼古丁
(R)-DRF053二盐酸盐
(5E)-5-[(2,5-二甲基-1-吡啶-3-基-吡咯-3-基)亚甲基]-2-亚磺酰基-1,3-噻唑烷-4-酮
(5-溴-3-吡啶基)[4-(1-吡咯烷基)-1-哌啶基]甲酮
(5-氨基-6-氰基-7-甲基[1,2]噻唑并[4,5-b]吡啶-3-甲酰胺)
(2S,2'S)-(-)-[N,N'-双(2-吡啶基甲基]-2,2'-联吡咯烷双(乙腈)铁(II)六氟锑酸盐
(2S)-2-[[[9-丙-2-基-6-[(4-吡啶-2-基苯基)甲基氨基]嘌呤-2-基]氨基]丁-1-醇
(2R,2''R)-(+)-[N,N''-双(2-吡啶基甲基)]-2,2''-联吡咯烷四盐酸盐
(1'R,2'S)-尼古丁1,1'-Di-N-氧化物
黄色素-37
麦斯明-D4
麦司明
麝香吡啶
鲁非罗尼
鲁卡他胺
高氯酸N-甲基甲基吡啶正离子
高氯酸,吡啶
高奎宁酸
马来酸溴苯那敏
马来酸氯苯那敏-D6
马来酸左氨氯地平
顺式-双(异硫氰基)(2,2'-联吡啶基-4,4'-二羧基)(4,4'-二-壬基-2'-联吡啶基)钌(II)
顺式-二氯二(4-氯吡啶)铂
顺式-二(2,2'-联吡啶)二氯铬氯化物
顺式-1-(4-甲氧基苄基)-3-羟基-5-(3-吡啶)-2-吡咯烷酮
顺-双(2,2-二吡啶)二氯化钌(II) 水合物
顺-双(2,2'-二吡啶基)二氯化钌(II)二水合物
顺-二氯二(吡啶)铂(II)
顺-二(2,2'-联吡啶)二氯化钌(II)二水合物
韦德伊斯试剂
非那吡啶
非洛地平杂质C
非洛地平
非戈替尼
非布索坦杂质66
非尼拉朵
非尼拉敏
雷索替丁
阿雷地平
阿瑞洛莫
阿扎那韦中间体
阿培利司N-6
阿伐曲波帕杂质40
间硝苯地平
间-硝苯地平
镉,二碘四(4-甲基吡啶)-
锌,二溴二[4-吡啶羧硫代酸(2-吡啶基亚甲基)酰肼]-