S-三唑并[4,3-a]喹啉 | 235-06-3
中文名称
S-三唑并[4,3-a]喹啉
中文别名
1,2,4-噻唑o[4,3-a]喹啉;1,2,4-三偶氮[4,3]喹啉
英文名称
1,2,4-triazolo[4,3-a]-quinoline
英文别名
naphtriazole;s-Triazolo<4,3-a>chinolin;[1,2,4]Triazolo[4,3-a]quinoline
CAS
235-06-3
化学式
C10H7N3
mdl
MFCD00004949
分子量
169.186
InChiKey
PIRYKGLQLCKQPG-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:175-177 °C
-
沸点:288.47°C (rough estimate)
-
密度:1.2396 (rough estimate)
-
稳定性/保质期:
在常温常压下保持稳定,应避免与不相容的材料接触。
计算性质
-
辛醇/水分配系数(LogP):2.5
-
重原子数:13
-
可旋转键数:0
-
环数:3.0
-
sp3杂化的碳原子比例:0.0
-
拓扑面积:30.2
-
氢给体数:0
-
氢受体数:2
安全信息
-
安全说明:S24/25
-
海关编码:2933990090
-
储存条件:密封保存,应储存在阴凉干燥的仓库中。
SDS
| Name: | S-Triazolo(4 3-a)Quinoline Material Safety Data Sheet |
| Synonym: | None |
| CAS: | 235-06-3 |
Synonym:None
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 235-06-3 | S-triazolo(4,3-a)Quinoline | ca 100 | 205-943-3 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation. The toxicological properties of this material have not been fully investigated.
Skin:
May cause skin irritation. The toxicological properties of this material have not been fully investigated.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media:
Use agent most appropriate to extinguish fire. Use water spray, dry chemical, carbon dioxide, or appropriate foam.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Clean up spills immediately, observing precautions in the Protective Equipment section. Sweep up, then place into a suitable container for disposal. Avoid generating dusty conditions. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Use adequate ventilation to keep airborne concentrations low.
Exposure Limits CAS# 235-06-3: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to prevent skin exposure.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Powder
Color: white to light yellow
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: 175.00 - 177.00 deg C
Autoignition Temperature: Not available.
Flash Point: Not available.
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density:
Molecular Formula: C10H7N3
Molecular Weight: 169.19
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, dust generation, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, oxides of nitrogen, irritating and toxic fumes and gases, carbon dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 235-06-3: XZ6158051 LD50/LC50:
Not available.
Carcinogenicity:
S-triazolo(4,3-a)Quinoline - Not listed by ACGIH, IARC, or NTP.
Other:
See actual entry in RTECS for complete information.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
S 28A After contact with skin, wash immediately with
plenty of water.
S 37 Wear suitable gloves.
S 45 In case of accident or if you feel unwell, seek
medical advice immediately (show the label where
possible).
WGK (Water Danger/Protection)
CAS# 235-06-3: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 235-06-3 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 235-06-3 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— 1-Chlor-s-triazolo<4,3-a>chinolin 41493-50-9 C10H6ClN3 203.631
反应信息
-
作为反应物:描述:参考文献:名称:Agent for the control of plant-pathogenic organisms摘要:使用和组合物包括用于控制植物病原体的特定s-三唑(4,3-a)喹啉化合物的方法,其中一些被声明为新化合物。公开号:US03953457A1
-
作为产物:描述:参考文献:名称:使用DMF及其衍生物作为单碳源的无金属[4 + 1]和[5 + 1]环化反应制备杂环摘要:1,2,4-三唑并[3,4- a ]吡啶和相关的杂环以及取代的三嗪是在各种药物和农用化学试剂中普遍发现的支架。在这里,我们报告了一种高效且实用的方法,该方法使用DMF及其衍生物进行[4 + 1]和[5 + 1]环化反应,以制备这些杂环。这种无金属的反应利用了贮存稳定的DMF作为溶剂和碳供体,使用咪唑氯化物作为催化剂的优势,温和的反应条件可耐受广泛的底物范围并替代。制备的3-未取代的1,2,4-三唑并[3,4- a ]吡啶和衍生物允许在3-位进一步引入各种官能团。DOI:10.1016/j.tetlet.2020.151844
文献信息
-
TRICYCLIC INHIBITORS OF 5-LIPOXYGENASE申请人:Hutchinson John Howard公开号:US20070173508A1公开(公告)日:2007-07-26Described herein are compounds and pharmaceutical compositions containing such compounds, which inhibit the activity of 5-lipoxygenase (5-LO). Also described herein are methods of using such 5-LO inhibitors, alone and in combination with other compounds, for treating respiratory, cardiovascular, and other leukotriene-dependent or leukotriene mediated conditions, diseases, or disorders.
-
Nitroalkanes as electrophiles: synthesis of triazole-fused heterocycles with neuroblastoma differentiation activity作者:Nicolai A. Aksenov、Alexander V. Aksenov、Nikita K. Kirilov、Nikolai A. Arutiunov、Dmitrii A. Aksenov、Vladimir Maslivetc、Zhenze Zhao、Liqin Du、Michael Rubin、Alexander KornienkoDOI:10.1039/d0ob01007c日期:——2-hydrazinylquinolines, 2-hydrazinylpyridines and bis-2,4-dihydrazinylpyrimidines in polyphosphoric acid (PPA) affording 1,2,4-triazolo[4,3-a]quinolines, 1,2,4-triazolo[4,3-a]pyridines and bis[1,2,4]triazolo[4,3-a:4′,3′-c]pyrimidines, respectively. The reaction expands the scope of heterocyclic annulations involving phosphorylated nitronates, believed to be the electrophilic intermediates formed from nitroalkanes
-
[EN] COMPOUNDS CONTAINING A N-HETEROARYL MOIETY LINKED TO FUSED RING MOIETIES FOR THE INHIBITION OF NAD(P)H OXIDASES AND PLATELET ACTIVATION<br/>[FR] COMPOSES COMPRENANT UNE FRACTION N-HETEROARYLE LIEE A DES FRACTIONS DE NOYAU FUSIONNEES ET DESTINES A L'INHIBITION DES NAD(P)H OXYDASES ET DE L'ACTIVATION DE PLAQUETTES申请人:VASOPHARM BIOTECH GMBH公开号:WO2005111041A1公开(公告)日:2005-11-24The invention relates to compounds containing a N-heteroaryl moiety, which is linked via oxygen, sulfur or nitrogen, or via a methylene bridge and oxygen, sulfur or nitrogen to a fused ring moiety, in particular to the 1,2,3-triazolo[4,5d]pyrimidine-7-yl radical. The invention also relates to a process for the preparation of said compounds and the use thereof in drugs for the treatment of NAD(P)H oxidases-related diseases and disorders and inhibition of platelet activation.
-
Triazoloquinoline derivatives申请人:MAY & BAKER LIMITED公开号:EP0174833A2公开(公告)日:1986-03-19Triazoloquinoline derivatives of the general formula: wherein R' represents a 1,2,4-triazolo[4,3-a]quinoline group which is unsubstituted or substituted by one or more substituents R4 [which may be the same or different and each represents a halogen atom or an amino, carboxy, cyano, nitro, hydroxy, formyl, trifluoromethyl, aryl, aryloxy, arylthio, benzyloxycarbonylamino, sulphamoyl, tetrazol-5-yl, carbamoyl, thiocarbamoyl, arylcarbamoyl or aroyl group, or a straight- or branched-chain alkyl group containing froml to 6 carbon atoms, or a straight- or branched-chain alkoxy, alkylthio, alkylsulphinyl, alkylsulphonyl, alkylamino, alkylsulphamoyl or arylalkyl group containing from 1 to 6 carbon atoms in the alkyl moiety, a straight- or branched-chain alkanoyl, alkoxycarbonyl, alkoxycarbonylamino, alkylcarbamoyl or alkanoylamino group containing from 2 to 6 carbon atoms, an N-benzyloxycarbonyl-N-alkylamino group wherein the alkylamino moiety is straight- or branched-chain and contains from 1 to 6 carbon atoms, or a dialkylsulphamoyl, dialkylamino or dialkylcarbamoyl group wherein the alkyl moieties may be straight or branched and may each contain from 1 to 6 carbon atoms and may be linked together to form a ring], R7 and R3 may be the same or different and each represents a cyano group, a straight- or branched-chain alkoxycarbonyl group containing from 2 to 6 carbon atoms. or a phenylsulphonyl group which may be unsubstituted or substituted by one or more substituents R4 (as hereinbefore defined), aryl groups and moieties and aroyl groups within the definition of R4 optionally bearing one or more substituents, and n represents 1 or 2 or, when an aryl group or moiety or an aroyl group represented by R4 carries one or more substituents - NHCH=CR2R3 (wherein R2 and R3 are as hereinbefore defined), n represents 0, 1 or 2, and salts thereof, possess useful pharmacological properties.通式的三唑并喹啉衍生物: 其中 R'代表未被取代或被一个或多个取代基 R4 取代的 1,2,4-三唑并[4,3-a]喹啉基团[这些取代基可以相同或不同,且各自代表卤素原子或氨基、羧基、氰基、硝基、羟基、甲酰基、三氟甲基、芳基、芳氧基、芳硫基、苄氧羰基氨基、磺酰胺基、四唑-5-基、氨基甲酰基、硫代氨基甲酰基、芳基氨基甲酰基或芳基基团],或直链基团、四唑-5-基、氨基甲酰基、硫代氨基甲酰基、芳基氨基甲酰基或芳基,或含有 1 至 6 个碳原子的直链或支链烷基,或在烷基中含有 1 至 6 个碳原子的直链或支链烷氧基、烷硫基、烷基亚磺酰基、烷基磺酰基、烷基氨基、烷基磺酰基或芳基烷基、直链或支链烷酰基、烷氧羰基、烷氧羰基氨基、烷基氨基甲酰基或含有 2 至 6 个碳原子的烷酰氨基、N-苄氧羰基-N-烷基氨基(其中烷基氨基分子为直链或支链且含有 1 至 6 个碳原子)或二烷基磺酰基、二烷基氨基或二烷基氨基甲酰基,其中的烷基可以是直链或支链,每个可含有 1 至 6 个碳原子,并可连接在一起形成一个环],R7 和 R3 可以相同或不同,每个代表一个氰基、一个含有 2 至 6 个碳原子的直链或支链烷氧基羰基。或苯基磺酰基,该基可以未被取代或被一个或多个取代基 R4(如前所述)取代,R4 定义范围内的芳基和分子以及芳烷基可选择带有一个或多个取代基,且 n 代表 1 或 2 或、当 R4 所代表的芳基或分子或芳基带有一个或多个取代基-NHCH=CR2R3(其中 R2 和 R3 如前定义)时,n 代表 0、1 或 2 及其盐类具有有用的药理特性。
-
ORGANIC COMPOUNDS CONTAINING SILICON OR GERMANIUM, TRANSITION METAL COMPLEXES, CATALYST FOR $g(a)-OLEFIN POLYMERIZATION, AND PROCESSES FOR PRODUCING $g(a)-OLEFIN POLYMERS申请人:MITSUBISHI CHEMICAL CORPORATION公开号:EP0985674A1公开(公告)日:2000-03-15An alkali metal- or alkali earth metal-containing organic compound (i) is reacted with a leaving group- and silicon- or germanium-containing compound (ii) in the presence of a nitrogen-containing aromatic heterocyclic compound. By such a method, it is possible to produce a silicon- or germanium-containing organic compound, for example, a cyclopentadienyl compound cross-linked by a silicon atom or a germanium atom, typically a cyclopentadienyl compound which is substituted with a substituted or unsubstituted silyl or germyl group, for a short time with a high yield.
表征谱图
-
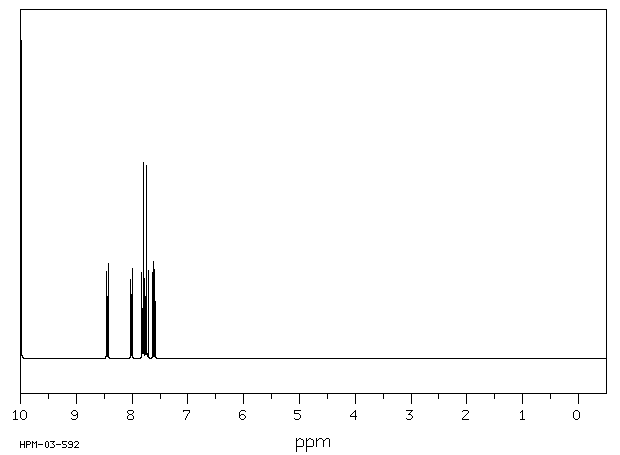
氢谱1HNMR
-
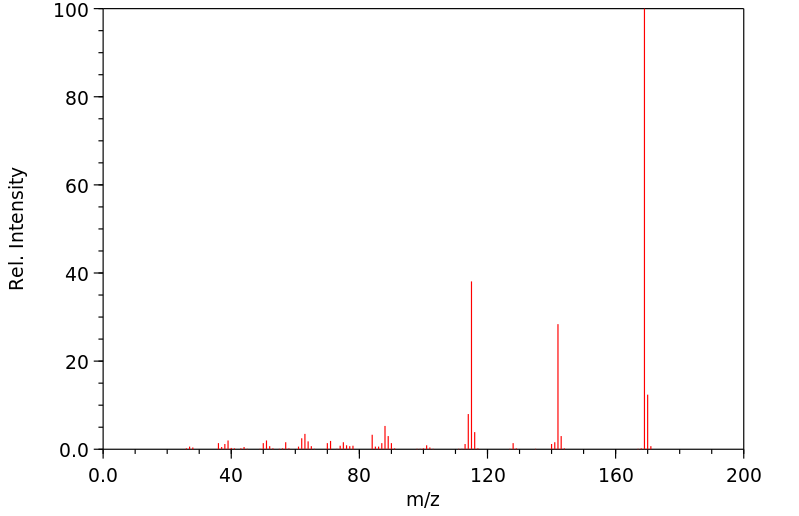
质谱MS
-
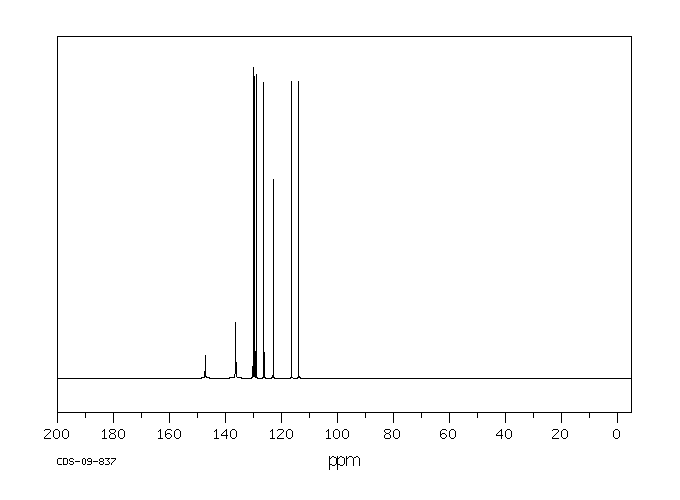
碳谱13CNMR
-
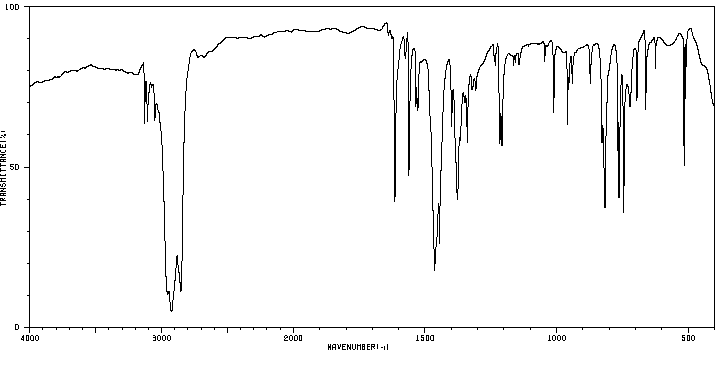
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-4-(叔丁基)-2-(喹啉-2-基)-4,5-二氢噁唑
(SP-4-1)-二氯双(喹啉)-钯
(E)-2-氰基-3-[5-(2,5-二氯苯基)呋喃-2-基]-N-喹啉-8-基丙-2-烯酰胺
(8α,9S)-(+)-9-氨基-七氢呋喃-6''-醇,值90%
(6,7-二甲氧基-4-(3,4,5-三甲氧基苯基)喹啉)
(1-羟基-5-硝基-8-氧代-8,8-dihydroquinolinium)
黄尿酸 8-甲基醚
麻保沙星EP杂质D
麻保沙星EP杂质B
麻保沙星EP杂质A
麦角腈甲磺酸盐
麦角腈
麦角灵
麦皮星酮
麦特氧特
高铁试剂
高氯酸3-苯基[1,3]噻唑并[3,2-f]5-氮杂菲-4-正离子
马波沙星EP杂质F
马波沙星
马来酸茚达特罗杂质
马来酸茚达特罗
马来酸维吖啶
马来酸来那替尼
马来酸四甲基铵
香草木宁碱
颜料红R-122
颜料红210
颜料红
顺式-苯并(f)喹啉-7,8-二醇-9,10-环氧化物
顺式-(alphaR)-N-(4-氯苯基)-4-(6-氟-4-喹啉基)-alpha-甲基环己烷乙酰胺
非那沙星
非那沙星
青花椒碱
青色素863
雷西莫特
隐花青
阿莫地喹-d10
阿莫地喹
阿莫吡喹N-氧化物
阿美帕利
阿米诺喹
阿立哌唑溴代杂质
阿立哌唑杂质B
阿立哌唑杂质38
阿立哌唑杂质1750
阿立哌唑杂质13
阿立哌唑杂质
阿立哌唑杂质
阿尔马尔
阿加曲班杂质43