4-(2-噻吩基)丁酸 | 4653-11-6
中文名称
4-(2-噻吩基)丁酸
中文别名
4-(2-噻嗯基)丁酸;2-噻吩丁酸;4-(2-噻吩)丁酸;4-2-噻吩丁酸;4-(2-噻嗯基)丁酸
英文名称
2-thiophenebutyric acid
英文别名
4-(2-Thienyl)butyric acid;4-(thiophen-2-yl)butanoic acid;4-thiophen-2-ylbutanoic acid
CAS
4653-11-6
化学式
C8H10O2S
mdl
MFCD00005463
分子量
170.232
InChiKey
VYTXLSQVYGNWLV-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:13.5-15 °C
-
沸点:122°C 0,3mm
-
密度:1.169 g/mL at 25 °C(lit.)
-
闪点:>230 °F
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.8
-
重原子数:11
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.375
-
拓扑面积:65.5
-
氢给体数:1
-
氢受体数:3
安全信息
-
危险等级:IRRITANT
-
危险品标志:Xi
-
安全说明:S26,S37/39
-
危险类别码:R36/37/38
-
WGK Germany:3
-
海关编码:2934999090
-
危险标志:GHS07
-
危险性描述:H315,H319,H335
-
危险性防范说明:P261,P305 + P351 + P338
-
包装等级:III
-
危险类别:8
-
危险品运输编号:3265
-
储存条件:请将药品存放在避光、通风干燥的地方,并密封保存。
SDS
| Name: | 4-(2-Thienyl)butyric Acid 98% Material Safety Data Sheet |
| Synonym: | 2-Thiophenebutyric Acid |
| CAS: | 4653-11-6 |
Synonym:2-Thiophenebutyric Acid
Section 2 - COMPOSITION, INFORMATION ON INGREDIENTS
| CAS# | Chemical Name | content | EINECS# |
| 4653-11-6 | 4-(2-Thienyl)butyric Acid | 98% | 225-090-0 |
Risk Phrases: None Listed.
Section 3 - HAZARDS IDENTIFICATION
EMERGENCY OVERVIEW
The toxicological properties of this material have not been fully investigated.
Potential Health Effects
Eye:
May cause eye irritation.
Skin:
May cause skin irritation.
Ingestion:
May cause irritation of the digestive tract. The toxicological properties of this substance have not been fully investigated.
Inhalation:
May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Chronic:
No information found.
Section 4 - FIRST AID MEASURES
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.
Skin:
Get medical aid. Flush skin with plenty of water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse.
Ingestion:
Never give anything by mouth to an unconscious person. Get medical aid. Do NOT induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water.
Inhalation:
Remove from exposure and move to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid.
Notes to Physician:
Section 5 - FIRE FIGHTING MEASURES
General Information:
As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Containers may explode in the heat of a fire. Vapors may be heavier than air.
They can spread along the ground and collect in low or confined areas. Runoff from fire control or dilution water may cause pollution.
Extinguishing Media:
Use water spray to cool fire-exposed containers. Use agent most appropriate to extinguish fire.
Section 6 - ACCIDENTAL RELEASE MEASURES
General Information: Use proper personal protective equipment as indicated in Section 8.
Spills/Leaks:
Absorb spill with inert material (e.g. vermiculite, sand or earth), then place in suitable container. Avoid runoff into storm sewers and ditches which lead to waterways. Clean up spills immediately, observing precautions in the Protective Equipment section. Provide ventilation.
Section 7 - HANDLING and STORAGE
Handling:
Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation.
Storage:
Keep container closed when not in use. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Section 8 - EXPOSURE CONTROLS, PERSONAL PROTECTION
Engineering Controls:
Facilities storing or utilizing this material should be equipped with an eyewash facility and a safety shower. Use process enclosure, local exhaust ventilation, or other engineering controls to control airborne levels.
Exposure Limits CAS# 4653-11-6: Personal Protective Equipment Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.
Skin:
Wear appropriate protective gloves to prevent skin exposure.
Clothing:
Wear appropriate protective clothing to minimize contact with skin.
Respirators:
Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
Section 9 - PHYSICAL AND CHEMICAL PROPERTIES
Physical State: Liquid
Color: clear slight brown
Odor: Not available.
pH: Not available.
Vapor Pressure: Not available.
Viscosity: Not available.
Boiling Point: Not available.
Freezing/Melting Point: Not available.
Autoignition Temperature: Not available.
Flash Point: > 112 deg C (> 233.60 deg F)
Explosion Limits, lower: Not available.
Explosion Limits, upper: Not available.
Decomposition Temperature:
Solubility in water:
Specific Gravity/Density: 1.1690g/cm3
Molecular Formula: C8H10O2S
Molecular Weight: 170.23
Section 10 - STABILITY AND REACTIVITY
Chemical Stability:
Stable under normal temperatures and pressures.
Conditions to Avoid:
Incompatible materials, excess heat, strong oxidants.
Incompatibilities with Other Materials:
Oxidizing agents.
Hazardous Decomposition Products:
Carbon monoxide, irritating and toxic fumes and gases, carbon dioxide, sulfur oxides (SOx), including sulfur oxide and sulfur dioxide.
Hazardous Polymerization: Has not been reported
Section 11 - TOXICOLOGICAL INFORMATION
RTECS#:
CAS# 4653-11-6 unlisted.
LD50/LC50:
Not available.
Carcinogenicity:
4-(2-Thienyl)butyric Acid - Not listed by ACGIH, IARC, or NTP.
Section 12 - ECOLOGICAL INFORMATION
Section 13 - DISPOSAL CONSIDERATIONS
Dispose of in a manner consistent with federal, state, and local regulations.
Section 14 - TRANSPORT INFORMATION
IATA
Not regulated as a hazardous material.
IMO
Not regulated as a hazardous material.
RID/ADR
Not regulated as a hazardous material.
Section 15 - REGULATORY INFORMATION
European/International Regulations
European Labeling in Accordance with EC Directives
Hazard Symbols: Not available.
Risk Phrases:
Safety Phrases:
S 24/25 Avoid contact with skin and eyes.
WGK (Water Danger/Protection)
CAS# 4653-11-6: No information available.
Canada
None of the chemicals in this product are listed on the DSL/NDSL list.
CAS# 4653-11-6 is not listed on Canada's Ingredient Disclosure List.
US FEDERAL
TSCA
CAS# 4653-11-6 is not listed on the TSCA inventory.
It is for research and development use only.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
用途
医药中间体
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 —— 4-[2]thienyl-butyric acid amide 4653-12-7 C8H11NOS 169.247 —— 4-(thiophen-2-yl)butanenitrile 28424-69-3 C8H9NS 151.232 3-(2-噻吩甲酰基)丙酸 4-oxo-4-thiophen-2-yl-butyric acid 4653-08-1 C8H8O3S 184.216 —— methyl 4-oxo-4-(2-thienyl)butanoate 87702-21-4 C9H10O3S 198.243 3-(2-噻吩基)-1-丙醇 3-thiophen-2-yl-propan-1-ol 19498-72-7 C7H10OS 142.222 3-(噻吩-2-基)丙基溴 2-(3-bromopropyl)thiophene 30134-51-1 C7H9BrS 205.118 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 4-(2-噻吩基)丁酸甲酯 methyl 4-(thiophen-2-yl)butanoate 20828-66-4 C9H12O2S 184.259 —— ethyl 4-(thiophen-2-yl)butanoate 91950-17-3 C10H14O2S 198.286 —— 4-(thiophen-2-yl)butan-1-ol 14330-41-7 C8H12OS 156.249 —— 5-thiophen-2-ylpentan-2-one 71777-99-6 C9H12OS 168.26 —— tert-butyl 4-(thiophen-2-yl)butanoate —— C12H18O2S 226.34 4-(5-溴-2-噻吩)丁酸 4-<5-Brom-2-thienyl>-buttersaeure 89980-93-8 C8H9BrO2S 249.128 —— 4-(5-chloro-thiophen-2-yl)-butyric acid 22168-06-5 C8H9ClO2S 204.677 —— 4-[2]thienyl-butyric acid amide 4653-12-7 C8H11NOS 169.247 4-(2-噻吩基)丁酰氯 4-(2-thienyl)butanoyl chloride 58372-62-6 C8H9ClOS 188.678 —— 6-[2]thienyl-hexan-3-one 855919-75-4 C10H14OS 182.287 —— 4-(2-thienyl)-butylamine 28424-67-1 C8H13NS 155.264 —— N-Methyl-4-(thiophene-2-yl)butyramide 165532-49-0 C9H13NOS 183.274 —— 4-(5-formyl-thiophen-2-yl)-butyric acid methyl ester 91061-69-7 C10H12O3S 212.269 —— 4-[5-(hydroxyimino-methyl)-[2]thienyl]-butyric acid 98996-54-4 C9H11NO3S 213.257 —— 2-(3-chloropropyl)thiophene 20906-17-6 C7H9ClS 160.667 3-(噻吩-2-基)丙基溴 2-(3-bromopropyl)thiophene 30134-51-1 C7H9BrS 205.118 2-(4-苯基-丁基)-噻吩 2-(4-phenylbutyl)thiophene 148112-00-9 C14H16S 216.347 —— 3-(2-thienyl)propylisocyanate 76197-88-1 C8H9NOS 167.232 - 1
- 2
反应信息
-
作为反应物:描述:4-(2-噻吩基)丁酸 在 吡啶 、 氢氧化钾 、 三氯化铝 、 氯化亚砜 、 乙醚 、 一水合肼 、 二乙二醇 作用下, 生成 1-(4,5,6,7-tetrahydro-benzo[b]thiophen-2-yl)-ethanone参考文献:名称:Cagniant; Cagniant, Bulletin de la Societe Chimique de France, 1953, p. 62,68, 713, 717摘要:DOI:
-
作为产物:描述:参考文献:名称:[EN] SUBSTITUTED PYRIDAZINE PHENOL DERIVATIVES
[FR] DÉRIVÉS DE PYRIDAZINE PHÉNOL SUBSTITUÉS
[ZH] 取代的哒嗪苯酚类衍生物摘要:一种取代的哒嗪苯酚类衍生物及其制备方法,具体涉及式(VI)所示化合物及其药学上可接受的盐,可用作NLRP3抑制剂。公开号:WO2022166890A1
文献信息
-
NOVEL COMPOUNDS AS CANNABINOID RECEPTOR LIGANDS AND USES THEREOF申请人:Florjancic S. Alan公开号:US20080058335A1公开(公告)日:2008-03-06The present invention relates to compounds of formula (I), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1 , R 2 , R 3 , and L 1 are defined in the specfication, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions. The present invention also relates to compounds of formula (II), or pharmaceutical salts, prodrugs, salts of prodrugs, or combinations thereof, wherein R 1a , R 2a and (Rx)n are as defined in the specification, compositions comprising such compounds, and methods of treating conditions and disorders using such compounds and compositions.本发明涉及式(I)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1 ,R 2 ,R 3 和L 1 在规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。本发明还涉及式(II)的化合物,或药用盐、前药、前药的盐或其组合物, 其中R 1a ,R 2a 和(Rx)n如规范中定义,包含这种化合物的组合物,以及使用这种化合物和组合物治疗疾病和疾病的方法。
-
Design and synthesis of a library of tertiary amides: Evaluation as mimetics of the melanocortins’ active core作者:Felikss Mutulis、Jana Kreicberga、Sviatlana Yahorava、Ilze Mutule、Larisa Borisova-Jan、Aleh Yahorau、Ruta Muceniece、Sandra Azena、Santa Veiksina、Ramona PetrovskaDOI:10.1016/j.bmc.2007.06.003日期:2007.9.1acceptable results. According to this approach an efficient formation of Schiff bases was achieved in the presence of TiCl(4). Substances were isolated by reversed phase chromatography; in some cases isomers were additionally separated by chiral chromatography on Chirobiotic T. When tested on human recombinant melanocortin receptors all the tertiary amides showed some binding affinities; for the highest affinity在固相上制备了201种叔酰胺。将二胺偶联到活化的羧化王聚合物上,并使所得的聚合物取代的苄氧羰基保护的二胺与原甲酸三甲酯中的醛或酮反应,得到树脂连接的席夫碱。然后将偶联的树脂通过在4%的乙酸/原甲酸三甲酯中的氰基硼氢化钠还原为仲胺,然后在PyBroP和二异丙基乙胺的存在下用羧酸酰化。在清除剂(主要是1,2-乙二硫醇)存在下,通过三氟乙酸从树脂上裂解叔酰胺。当制备吲哚衍生物时,与连接体片段发生平行烷基化,得到2-(4-羟基苄基)-吲哚的衍生物作为副产物。在上述方法未给出可接受的结果的情况下,标题化合物的溶液合成或液相/固相混合制备被证明是有利的。根据这种方法,在TiCl(4)存在的情况下实现了席夫碱的有效形成。物质经反相色谱分离。在某些情况下,还可以通过在Chirobiotic T上进行手性色谱分离来分离异构体。在人重组黑皮质素受体上进行测试时,所有叔酰胺都表现出一定的结合亲和力。对于具有最高
-
Reaction Discovery by Using a Sandwich Immunoassay作者:Julia Quinton、Sergii Kolodych、Manon Chaumonet、Valentina Bevilacqua、Marie-Claire Nevers、Hervé Volland、Sandra Gabillet、Pierre Thuéry、Christophe Créminon、Frédéric TaranDOI:10.1002/anie.201201451日期:2012.6.18Mmm, a reaction sandwich…︁ Using an immunoassay‐based technique able to monitor any kind of cross‐coupling reaction, a systematic and rapid evaluation of a large panel of random reactions was carried out. This approach led to the discovery of two new copper‐promoted reactions: a desulfurization reaction of thioureas leading to isoureas and a cyclization reaction leading to thiazole derivatives from
-
Ruthenium(II)-Catalyzed Enantioselective γ-Lactams Formation by Intramolecular C–H Amidation of 1,4,2-Dioxazol-5-ones作者:Qi Xing、Chun-Ming Chan、Yiu-Wai Yeung、Wing-Yiu YuDOI:10.1021/jacs.9b00535日期:2019.3.6C-H insertion. By employing chiral diphenylethylene diamine (dpen) as ligands bearing electron-withdrawing arylsulfonyl substituents, the reactions occur with remarkable chemo- and enantioselectivities; the competing Curtius-type rearrangement was largely suppressed. Enantioselective nitrene insertion to allylic/propargylic C-H bonds was also achieved with remarkable tolerance to the C═C and C≡C bonds
-
N-substituted nonaryl-heterocyclic NMDA/NR2B antagonists
表征谱图
-
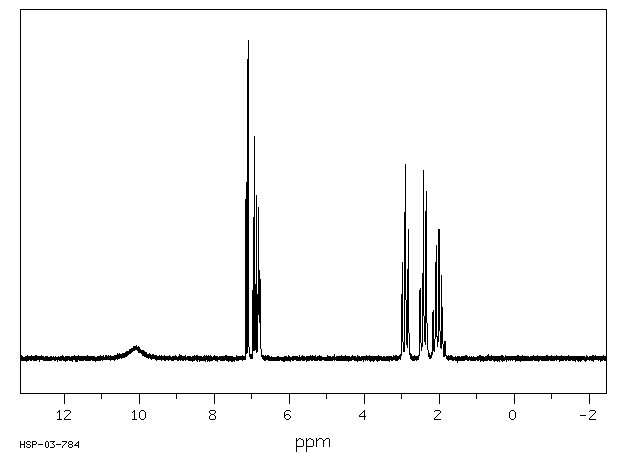
氢谱1HNMR
-
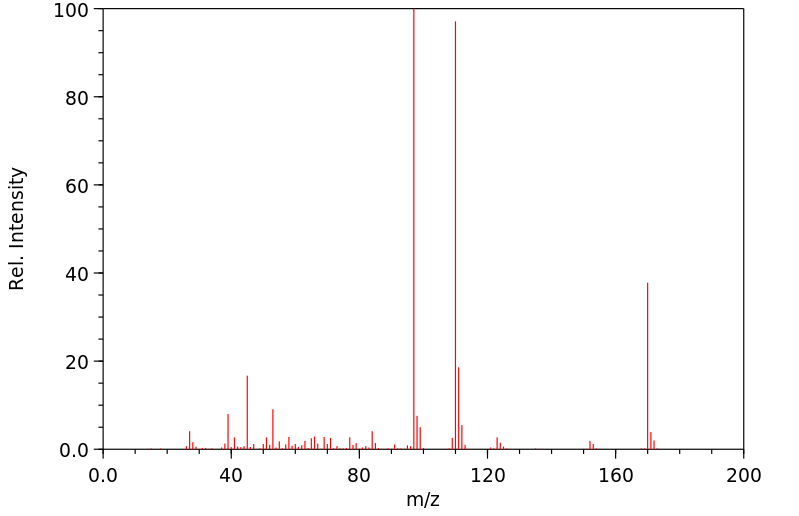
质谱MS
-
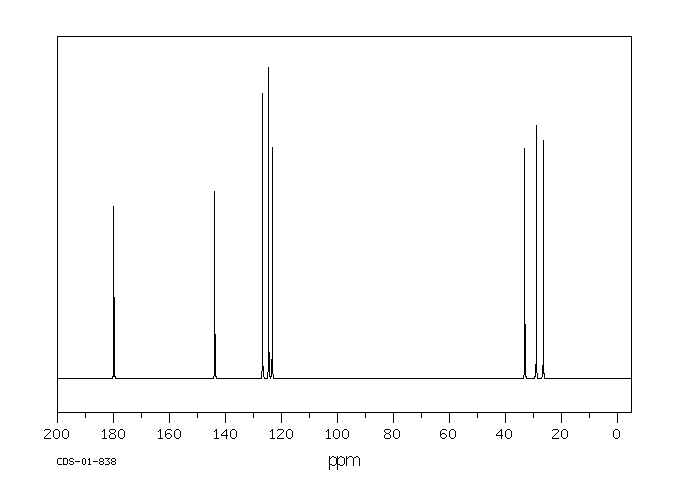
碳谱13CNMR
-
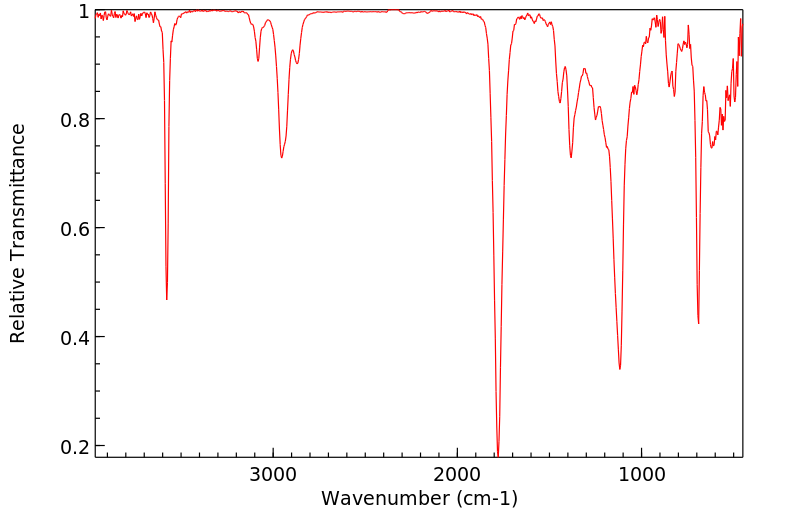
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
香薷二醇
顺式-1-(2-呋喃基)-1-戊烯
顺-1,2-二氰基-1,2-双(2,4,5-三甲基-3-噻吩基)乙烯
顺-1,2-(2-噻嗯基)二乙烯
雷尼替丁-N,S-二氧化物
雷尼替丁-N-氧化物
钴(II)双[(2-吡啶基甲基)(叔丁基二甲基甲硅烷基)酰胺]
西拉诺德
螺[环氧乙烷-2,3'-吡咯并[1,2-a]吡嗪]
萘并[2,1,8-def]喹啉
苯硫基溴化镁
苯甲酸,2-[[[7-[[(3.β.)-3-羟基-28-羰基羽扇-20(29)-烯-28-基]amino]庚基]氨基]羰基]
苍术素
羟胺,O-[4-(2-呋喃基)丁基]-
缩水甘油糠醚
紫苏烯
糠醛肟
糠醛氰醇的1-乙氧基乙基醚
糠醇-d2
糠醇
糠基硫醇-d2
糠基硫醇
糠基甲基硫醚
糠基氯
糠基氨基甲酸异丙酯
糠基丙基醚
糠基丙基二硫醚
糠基3-巯基-2-甲基丙酸酯
糠基-异戊基醚
糠基-异丁基醚
糠基 2-甲基-3-呋喃基二硫醚
磷杂茂
碘化N,N,N-三甲基丁烷-1-铵
硫酸异丙基糠酯
硫代磷酸O-糠基O-甲基S-(2-丙炔基)酯
硫代磷酸O-乙基O-糠基S-(2-丙炔基)酯
硫代甲酸S-糠酯
硫代噻吩甲酰基三氟丙酮
硫代乙酸糠酯
硫代丙酸糠酯
硒吩-3-羧酸酰肼
硅烷,三(1-甲基乙基)[(3-甲基-2-呋喃基)氧代]-
硅烷,[2-(3-呋喃基)乙烯基]三甲基-,(E)-
硅烷,(1,1-二甲基乙基)(2-呋喃基甲氧基)二甲基-
砷杂苯
甲酸糠酯
甲氧亚胺基呋喃乙酸铵盐
甲基糠基醚
甲基糠基二硫
甲基呋喃-2-基甲基氨基甲酸酯