5-苄氧基色胺 | 20776-45-8
中文名称
5-苄氧基色胺
中文别名
2-(5-苄氧基-1H-吲哚-3-基)-乙胺
英文名称
5-benzyloxytryptamine
英文别名
Ba 2810;2-(5-phenylmethoxy-1H-indol-3-yl)ethanamine
CAS
20776-45-8
化学式
C17H18N2O
mdl
MFCD00037972
分子量
266.343
InChiKey
WKPDXBXNJWWWGQ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:248-250 °C
-
沸点:480.5±35.0 °C(Predicted)
-
密度:1.193±0.06 g/cm3(Predicted)
-
溶解度:7.8 [ug/mL]
计算性质
-
辛醇/水分配系数(LogP):2
-
重原子数:20
-
可旋转键数:5
-
环数:3.0
-
sp3杂化的碳原子比例:0.176
-
拓扑面积:51
-
氢给体数:2
-
氢受体数:2
安全信息
-
海关编码:2933990090
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
Product Name: 2-(5-Benzyloxy-1h-indol-3-yl)-ethylamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-(5-Benzyloxy-1h-indol-3-yl)-ethylamine
CAS number: 20776-45-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C17H18N2O
Molecular weight: 266.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
Product Name: 2-(5-Benzyloxy-1h-indol-3-yl)-ethylamine
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
Section 3. Composition/information on ingredients.
Ingredient name: 2-(5-Benzyloxy-1h-indol-3-yl)-ethylamine
CAS number: 20776-45-8
Section 4. First aid measures
Skin contact: Immediately wash skin with copious amounts of water for at least 15 minutes while removing
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Ingestion: Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Store in closed vessels, refrigerated.
Storage:
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Appearance: Not specified
Boiling point: No data
No data
Melting point:
Flash point: No data
Density: No data
Molecular formula: C17H18N2O
Molecular weight: 266.3
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 5-苄氧基吲哚-3-乙酰胺 5-benzyloxyindole-3-acetamide 5933-28-8 C17H16N2O2 280.326 5-苄氧基吲哚-3-乙腈 5-benzyloxyindole-3-acetonitrile 2436-15-9 C17H14N2O 262.311 —— N-tert-Butoxycarbonyl-O-benzyl-5-hydroxytryptamin 53157-47-4 C22H26N2O3 366.46 3-(5-苄氧基-吲哚-3-基)-丙酸 3-(5-benzyloxy-indol-3-yl)-propionic acid 7394-76-5 C18H17NO3 295.338 5-羟基色胺 3-(2-aminoethyl)-1H-indol-5-ol 50-67-9 C10H12N2O 176.218 —— 3-(5-benzyloxy-indol-3-yl)-propionic acid methyl ester 101890-43-1 C19H19NO3 309.365 —— 5-formyloxy-N-methoxycarbonyltryptamine 329764-36-5 C13H14N2O4 262.265 5-苄氧基吲哚-3-乙醛酰胺 5-(Benzyloxy)indol-3-yloxylamid 22424-62-0 C17H14N2O3 294.31 5-苄氧基吲哚-3-甲醛 5-benzyloxyindole-3-carboxaldehyde 6953-22-6 C16H13NO2 251.285 —— 5-hydroxy-N-methoxycarbonyltryptamine 77549-09-8 C12H14N2O3 234.255 N-叔-丁基氧羰基血清素 tert-butyl 2-(5-hydroxy-1H-indol-3-yl)ethylcarbamate 53157-48-5 C15H20N2O3 276.335 5-苄氧基芦竹碱 5-benzyloxygramine 1453-97-0 C18H20N2O 280.37 —— 5-benzyloxy-1-formyl-N-methoxycarbonyltryptamine 284028-36-0 C20H20N2O4 352.39 —— 5-Benzyloxy-3-indol-glyoxylylchlorid 22424-61-9 C17H12ClNO3 313.74 5-苄氧基-3-(2-硝基乙烯基)-吲哚 5-Benzyloxy-3-(2-nitro-vinyl)-indole 69796-46-9 C17H14N2O3 294.31 5-苄氧基吲哚 5-benzyloxy-1H-indole 1215-59-4 C15H13NO 223.274 —— 1-formyl-5-hydroxy-N-methoxycarbonyltryptamine 185987-04-6 C13H14N2O4 262.265 5-苯甲氧基吲哚-2-羧酸 5-benzyloxy-1H-indole-2-carboxylic acid 6640-09-1 C16H13NO3 267.284 —— 1-hydroxy-Nb-methoxycarbonyltryptamine 180910-53-6 C12H14N2O3 234.255 —— 1-methoxy-Nb-methoxycarbonyltryptamine 69111-85-9 C13H16N2O3 248.282 - 1
- 2
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 N,N-二甲基-5-(苄氧基)-1H-吲哚-3-乙胺 [2-(5-benzyloxy-1H-indol-3-yl)ethyl]dimethylamine 25390-67-4 C19H22N2O 294.396 N-{2-[5-(苄氧基)-1H-吲哚-3-基]乙基}乙酰胺 N-acetyl-5-(benzyloxy)tryptamine 68062-88-4 C19H20N2O2 308.38 5-苄氧基吲哚-3-乙腈 5-benzyloxyindole-3-acetonitrile 2436-15-9 C17H14N2O 262.311 —— N-[2-(5-benzyloxyindol-3-yl)ethyl]succinamic acid 284028-37-1 C21H22N2O4 366.417 —— 5-Propoxytryptamine 3610-40-0 C13H18N2O 218.299 —— 5-acetylserotonin 46505-63-9 C12H14N2O2 218.255 —— methyl N-[2-(5-benzyloxyindol-3-yl)ethyl]succinamate —— C22H24N2O4 380.444 —— 3-(2-aminoethyl)-5-(n-pentyloxy)indole 171783-15-6 C15H22N2O 246.352 5-壬基氧基色胺草酸盐 5-(Nonyloxy)tryptamine 157798-12-4 C19H30N2O 302.46 5-羟基色胺 3-(2-aminoethyl)-1H-indol-5-ol 50-67-9 C10H12N2O 176.218 —— N-[2-(5-benzyloxyindol-3-yl)ethyl]succinimide —— C21H20N2O3 348.401 —— N-[2-(5-propoxy-1H-indol-3-yl)ethyl]acetamide 76290-75-0 C15H20N2O2 260.336 —— N-[2-(5-nonoxy-1H-indol-3-yl)ethyl]acetamide 176444-96-5 C21H32N2O2 344.497 —— N-[2-(5-heptoxy-1H-indol-3-yl)ethyl]acetamide 1026239-22-4 C19H28N2O2 316.444 N-乙酰-5-羟色胺 N-acetyl-5-hydroxytryptamine 1210-83-9 C12H14N2O2 218.255 —— N-{(S)-1-[2-(5-Benzyloxy-1H-indol-3-yl)-ethylcarbamoyl]-ethyl}-3-phenyl-propionamide 1076204-01-7 C29H31N3O3 469.583 —— bufobutanoic acid —— C14H16N2O4 276.292 —— 5-Benzyloxy-3-(2-phthalimino-ethyl)-indol 53157-45-2 C25H20N2O3 396.445 —— (2S)-N-[2-(5-phenylmethoxy-1H-indol-3-yl)ethyl]-1-(3-phenylpropanoyl)pyrrolidine-2-carboxamide 1076203-98-9 C31H33N3O3 495.621 —— 4-(4-{2-[5-Benzyloxy-2-(3,5-dimethyl-phenyl)-1H-indol-3-yl]-ethylamino}-butyl)-phenol 192771-98-5 C35H38N2O2 518.699 —— 2-[2-(5-benzyloxy-2-bromo-1H-indol-3-yl)ethyl]isoindole-1,3-dione 192182-48-2 C25H19BrN2O3 475.341 —— 3-[2-[4-(4-benzyloxyphenyl)butylamino]ethyl]-2-(3,5-dimethylphenyl)-1H-indol-5-ol 192773-95-8 C35H38N2O2 518.699 —— 4-(4-benzyloxyphenyl)-N-{2-[2-(3,5-dimethylphenyl)-5-hydroxy-1H-indol-3-yl]ethyl]butyramide 192773-94-7 C35H36N2O3 532.682 —— 2-Acetyl-6-benzyloxy-1-methyl-1,2,3,4-tetrahydro-2-carbolin 95701-83-0 C21H22N2O2 334.418 —— 3-(2-aminoethyl)-2-(3,5-dimethylphenyl)-1H-indol-5-ol 192773-93-6 C18H20N2O 280.37 —— N-[2-(5-hydroxy-1H-indol-3-yl)ethyl] (2S,3S)-3-({(S)-3-methyl-1-[(3-methylbutyl)carbamoyl]butyl}carbamoyl)-2-oxiranecarboxamide 140660-58-8 C25H36N4O5 472.585 —— [4-(4-benzyloxyphenyl)-butyl]-[2-[2-(3,5-dimethylphenyl)-5-hydroxy-1H-indol-3-yl]ethyl]carbamic acid benzyl ester 192773-96-9 C43H44N2O4 652.833 - 1
- 2
- 3
反应信息
-
作为反应物:参考文献:名称:790. 5-羟基吲哚的3-2'-氨基丙基-和3-2'-氨基丁基衍生物的合成,以及5-羟基色胺的替代合成摘要:DOI:10.1039/jr9580003887
-
作为产物:描述:5-羟基色胺 在 吡啶 、 sodium hydroxide 、 potassium carbonate 作用下, 以 甲醇 、 水 、 N,N-二甲基甲酰胺 为溶剂, 反应 56.0h, 生成 5-苄氧基色胺参考文献:名称:The Chemistry of Indoles. CIII. Simple Syntheses of Serotonin, N-Methylserotonin, Bufotenine, 5-Methoxy-N-methyltryptamine, Bufobutanoic Acid, N-(Indol-3-yl)methyl-5-methoxy-N-methyltryptamine, and Lespedamine Based on 1-Hydroxyindole Chemistry.摘要:本文描述了利用1-羟色胺的选择性亲核取代反应,合成新型简单物质的方法,这些物质包括:血清素(1a)、N-甲基血清素(1b)、蟾毒色胺(1c)、5-甲氧基-N-甲基色胺(2a)、蟾毒色酸(3a)、N-(吲哚-3-基)甲基-5-甲氧基-N-甲基色胺(4)和莱斯佩达明(5)。此外,还报道了有效的5-苄氧基色胺和1-甲氧基-2-氧吲哚合成方法。DOI:10.1248/cpb.49.87
文献信息
-
5-HT Reuptake Inhibitors with 5-HT<sub>1B/1D</sub> Antagonistic Activity: A New Approach toward Efficient Antidepressants作者:Lisa Matzen、Christoph van Amsterdam、Wilfried Rautenberg、Hartmut E. Greiner、Jürgen Harting、Christoph A. Seyfried、Henning BöttcherDOI:10.1021/jm9811054日期:2000.3.1block the sumatriptan-induced inhibition of potassium-evoked [(3)H]5-HT release. The 5-HT reuptake inhibition of the ureas determined in rat brain synaptosomes was found to be either increased or decreased as compared to the uncoupled indole derivatives indicating that the reuptake inhibition shown by the ureas is not only due to the indole part but also affected by the aniline moiety of the molecule. Among作为我们针对新型潜在抗抑郁药的研究计划的一部分,已制备了一系列不对称尿素,并将其评估为具有5-HT(1B / 1D)拮抗活性的5-HT再摄取抑制剂。这些化合物的设计基于各种吲哚衍生物(以前显示出抑制5-HT再摄取的作用)与三个不同的苯胺部分的偶联,而苯胺部分是已知的5-HT(1B / 1D)配体的一部分。分别在大鼠额叶皮层中使用[(125)I]碘氰基吲哚醇,在小腿纹状体中使用[(3)H] 5-HT以及在大鼠海马中使用[(3)H] 8-OH-DPAT作为放射性配体的结合实验,揭示了与5-HT(1A)和5-HT(1D)受体对许多化合物的亲和力相比,对5-HT(1B)受体的亲和力明显更高,其中4-(5-氟-1H-吲哚-3-基)哌啶-1-甲酸[4-甲氧基-3-(4-甲基哌嗪-1-基)苯基]酰胺(5),相应的4-氟-1H-吲哚-3-基类似物21a和相应的6-氟-1H-吲哚-3-基类似物21b。苯胺部
-
Asymmetric Dearomatization of Indoles through a Michael/Friedel-Crafts-Type Cascade To Construct Polycyclic Spiroindolines作者:Xiaohu Zhao、Xiaohua Liu、Hongjiang Mei、Jing Guo、Lili Lin、Xiaoming FengDOI:10.1002/anie.201410814日期:2015.3.23through a cascade reaction between 2‐isocyanoethylindole and alkylidene malonates catalyzed by a chiral N,N′‐dioxide/MgII catalyst. Fused polycyclic indolines containing three stereocenters were afforded in good yields with excellent diastereo‐ and enantioselectivities through a Michael/Friedel–Crafts/Mannich cascade. When 2‐substituted 2‐isocyanoethylindoles were used, spiroindoline derivatives were obtained
-
Adenosine Analogues as Selective Inhibitors of Glyceraldehyde-3-phosphate Dehydrogenase of <i>Trypanosomatidae</i> via Structure-Based Drug Design作者:Jerome C. Bressi、Christophe L. M. J. Verlinde、Alex M. Aronov、My Le Shaw、Sam S. Shin、Lisa N. Nguyen、Stephen Suresh、Frederick S. Buckner、Wesley C. Van Voorhis、Irwin D. Kuntz、Wim G. J. Hol、Michael H. GelbDOI:10.1021/jm000472o日期:2001.6.1N(6)-[1-(3-hydroxynaphthalene)methyl]-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (7m), N(6)-[1-(3-methoxynaphthalene)methyl]-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (9m), N(6)-(2-naphthalenemethyl)-2'-deoxy-2'-(3-methoxybenzamido)adenosine (11e), and N(6)-(2-naphthalenemethyl)-2'-deoxy-2'-(3,5-dimethoxybenzamido)adenosine (11m) demonstrated a 2- to 3-fold improvement over 6e and a 7100- to 25000-fold improvement在我们继续进行基于抗锥虫病药物的结构设计时,寄生虫选择性腺苷类似物被确定为低甘油三磷酸甘油醛脱氢酶(GAPDH)的抑制剂。布鲁氏锥虫,克氏锥虫,墨西哥利什曼原虫和人类GAPDH的晶体结构提供了NAD(+)的腺苷部分如何与蛋白质相互作用的详细信息,这有助于理解一系列腺苷类似物对各种氨基酸的亲和力。 GAPDH。从对铅化合物的萘甲基和苯甲酰胺取代基进行修饰的探索中,N(6)-(1-萘甲基)-2'-脱氧-2'-(3-甲氧基苯甲酰胺基)腺苷(6e),N(6)-(研究了取代的萘甲基)-2'-脱氧-2'-(取代的苯甲酰胺基)腺苷类似物。N(6)-(1-萘甲基)-2'-脱氧-2'-(3,5-二甲氧基苯甲酰胺基)腺苷(6m),N(6)-[1-(3-羟基萘)甲基] -2'-脱氧-2'-(3,5-二甲氧基苯甲酰胺基)腺苷(7m),N(6)-[1-(3-甲氧基萘)甲基] -2'-脱氧-2'-(3,5-二甲氧基苯甲酰胺基)腺苷(
-
Hydrogen‐Bonding‐Promoted Cascade Rearrangement Involving the Enlargement of Two Rings: Efficient Access to Polycyclic Quinoline Derivatives作者:Wen‐Bin Cao、Shijun Li、Meng‐Meng Xu、Haiyan Li、Xiao‐Ping Xu、Yu Lan、Shun‐Jun JiDOI:10.1002/anie.202008110日期:2020.11.23An efficient cascade reaction of tryptamine‐derived isocyanides with C,N‐cyclic azomethine imines is described. The polycyclic pyrrolo[2,3‐c]quinoline derivatives, which benefited from rearrangement process driven by hydrogen bonding, could be directly assembled in moderate to good yields (40–87 %) under metal‐free and mild conditions. This transformation involved four new heterocyclic rings formations
-
A Versatile Linkage Strategy for Solid-Phase Synthesis of <i>N</i>,<i>N</i>-Dimethyltryptamines and β-Carbolines作者:Tom Y. H. Wu、Peter G. SchultzDOI:10.1021/ol026729p日期:2002.11.1Various tryptamines are captured by a vinylsulfonylmethyl polystyrene resin, generating a safety-catch linkage. Beta-carbolines can be formed from 4 by a Pictet-Spengler reaction with the introduction of R(1). Tryptamine 4 can also be derivatized by acylation or copper-mediated coupling to introduce R(2). If X = Br, Suzuki coupling can be used to introduce R(3). After derivatization, the indole derivatives
表征谱图
-
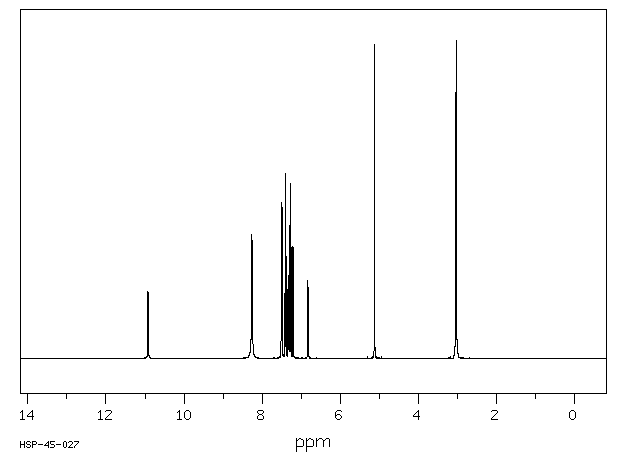
氢谱1HNMR
-
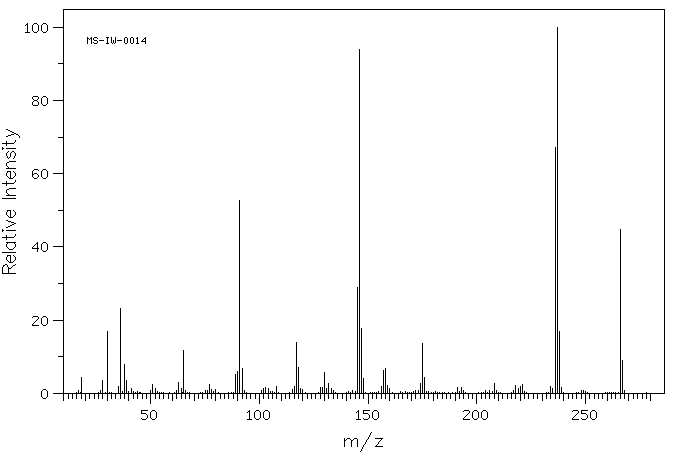
质谱MS
-
碳谱13CNMR
-
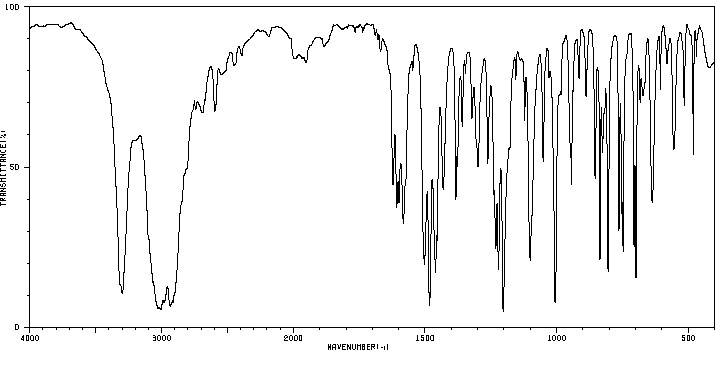
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(Z)-3-[[[2,4-二甲基-3-(乙氧羰基)吡咯-5-基]亚甲基]吲哚-2--2-
(S)-(-)-5'-苄氧基苯基卡维地洛
(R)-(+)-5'-苄氧基卡维地洛
(R)-卡洛芬
(N-(Boc)-2-吲哚基)二甲基硅烷醇钠
(E)-2-氰基-3-(5-(2-辛基-7-(4-(对甲苯基)-1,2,3,3a,4,8b-六氢环戊[b]吲哚-7-基)-2H-苯并[d][1,2,3]三唑-4-基)噻吩-2-基)丙烯酸
(4aS,9bR)-6-溴-2,3,4,4a,5,9b-六氢-1H-吡啶并[4,3-B]吲哚
(3Z)-3-(1H-咪唑-5-基亚甲基)-5-甲氧基-1H-吲哚-2-酮
(3Z)-3-[[[4-(二甲基氨基)苯基]亚甲基]-1H-吲哚-2-酮
(3R)-(-)-3-(1-甲基吲哚-3-基)丁酸甲酯
(3-氯-4,5-二氢-1,2-恶唑-5-基)(1,3-二氧代-1,3-二氢-2H-异吲哚-2-基)乙酸
齐多美辛
鸭脚树叶碱
鸭脚木碱,鸡骨常山碱
鲜麦得新糖
高氯酸1,1’-二(十六烷基)-3,3,3’,3’-四甲基吲哚碳菁
马鲁司特
马鞭草(VERBENAOFFICINALIS)提取物
马来酸阿洛司琼
马来酸替加色罗
顺式-ent-他达拉非
顺式-1,3,4,4a,5,9b-六氢-2H-吡啶并[4,3-b]吲哚-2-甲酸乙酯
顺式-(+-)-3,4-二氢-8-氯-4'-甲基-4-(甲基氨基)-螺(苯并(cd)吲哚-5(1H),2'(5'H)-呋喃)-5'-酮
靛青二磺酸二钾盐
靛藍四磺酸
靛红联二甲酚
靛红磺酸钠
靛红磺酸
靛红乙烯硫代缩酮
靛红-7-甲酸甲酯
靛红-5-磺酸钠
靛红-5-磺酸
靛红-5-硫酸钠盐二水
靛红-5-甲酸甲酯
靛红
靛玉红衍生物E804
靛玉红3'-单肟5-磺酸
靛玉红-3'-单肟
靛玉红
靛噻
青色素3联己酸染料,钾盐
雷马曲班
雷莫司琼杂质13
雷莫司琼杂质12
雷莫司琼杂质
雷替尼卜定
雄甾-1,4-二烯-3,17-二酮
阿霉素的代谢产物盐酸盐
阿贝卡尔
阿西美辛杂质3