DL-苯基丙氨酸甲酯盐酸盐 | 5619-07-8
中文名称
DL-苯基丙氨酸甲酯盐酸盐
中文别名
DL-蛋氨酸甲基磺酰氯
英文名称
methyl 2-amino-3-phenylpropanoate hydrochloride
英文别名
phenylalanine methyl ester hydrochloride;methyl phenylalaninate hydrochloride;D/L-phenylalanine methyl ester hydrochloride;(±)-phenylalanine methyl ester hydrochloride;L/D-phenylalanine methyl ester hydrochloride;Hydron;methyl 2-amino-3-phenylpropanoate;chloride
CAS
5619-07-8
化学式
C10H14NO2*Cl
mdl
MFCD00066113
分子量
215.68
InChiKey
SWVMLNPDTIFDDY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:158-162°C
-
溶解度:可溶于DMSO(少量)、甲醇(少量)
-
稳定性/保质期:
在常温常压下保持稳定
计算性质
-
辛醇/水分配系数(LogP):1.22
-
重原子数:14
-
可旋转键数:4
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:52.3
-
氢给体数:2
-
氢受体数:3
安全信息
-
安全说明:S22,S24/25,S26,S36/37/39
-
危险类别码:R36/37/38
-
危险性防范说明:P261,P305+P351+P338
-
危险性描述:H315,H319,H335
-
储存条件:-15℃ 下应避光、存于阴凉干燥处并密封保存。
SDS
Material Safety Data Sheet
Section 1. Identification of the substance
DL-phenylalanine methyl ester, HCl
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
DL-phenylalanine methyl ester, HCl
Ingredient name:
CAS number: 5619-07-8
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H13NO2.ClH
Molecular weight: 215.7
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
Section 1. Identification of the substance
DL-phenylalanine methyl ester, HCl
Product Name:
Synonyms:
Section 2. Hazards identification
Harmful by inhalation, in contact with skin, and if swallowed.
H315: Causes skin irritation
H319: Causes serious eye irritation
H335: May cause respiratory irritation
P261: Avoid breathing dust/fume/gas/mist/vapours/spray
Wear protective gloves/protective clothing/eye protection/face protection
P280:
P305+P351+P338: IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses if present
and easy to do – continue rinsing
P304+P340: IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for breathing
P405: Store locked up
Section 3. Composition/information on ingredients.
DL-phenylalanine methyl ester, HCl
Ingredient name:
CAS number: 5619-07-8
Section 4. First aid measures
Immediately wash skin with copious amounts of water for at least 15 minutes while removing
Skin contact:
contaminated clothing and shoes. If irritation persists, seek medical attention.
Eye contact: Immediately wash skin with copious amounts of water for at least 15 minutes. Assure adequate
flushing of the eyes by separating the eyelids with fingers. If irritation persists, seek medical
attention.
Inhalation: Remove to fresh air. In severe cases or if symptoms persist, seek medical attention.
Wash out mouth with copious amounts of water for at least 15 minutes. Seek medical attention.
Ingestion:
Section 5. Fire fighting measures
In the event of a fire involving this material, alone or in combination with other materials, use dry
powder or carbon dioxide extinguishers. Protective clothing and self-contained breathing apparatus
should be worn.
Section 6. Accidental release measures
Personal precautions: Wear suitable personal protective equipment which performs satisfactorily and meets local/state/national
standards.
Respiratory precaution: Wear approved mask/respirator
Hand precaution: Wear suitable gloves/gauntlets
Skin protection: Wear suitable protective clothing
Eye protection: Wear suitable eye protection
Methods for cleaning up: Mix with sand or similar inert absorbent material, sweep up and keep in a tightly closed container
for disposal. See section 12.
Environmental precautions: Do not allow material to enter drains or water courses.
Section 7. Handling and storage
Handling: This product should be handled only by, or under the close supervision of, those properly qualified
in the handling and use of potentially hazardous chemicals, who should take into account the fire,
health and chemical hazard data given on this sheet.
Storage: Store in closed vessels.
Section 8. Exposure Controls / Personal protection
Engineering Controls: Use only in a chemical fume hood.
Personal protective equipment: Wear laboratory clothing, chemical-resistant gloves and safety goggles.
General hydiene measures: Wash thoroughly after handling. Wash contaminated clothing before reuse.
Section 9. Physical and chemical properties
Not specified
Appearance:
Boiling point: No data
Melting point: No data
Flash point: No data
Density: No data
Molecular formula: C10H13NO2.ClH
Molecular weight: 215.7
Section 10. Stability and reactivity
Conditions to avoid: Heat, flames and sparks.
Materials to avoid: Oxidizing agents.
Possible hazardous combustion products: Carbon monoxide, nitrogen oxides, hydrogen chloride.
Section 11. Toxicological information
No data.
Section 12. Ecological information
No data.
Section 13. Disposal consideration
Arrange disposal as special waste, by licensed disposal company, in consultation with local waste
disposal authority, in accordance with national and regional regulations.
Section 14. Transportation information
Non-harzardous for air and ground transportation.
Section 15. Regulatory information
No chemicals in this material are subject to the reporting requirements of SARA Title III, Section
302, or have known CAS numbers that exceed the threshold reporting levels established by SARA
Title III, Section 313.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
DL-苯基丙氨酸甲酯盐酸盐具有光学活性,在医药、食品和农药等多个领域得到了广泛应用。
反应信息
-
作为反应物:描述:参考文献:名称:通过相应的酰胺和腈底物与多酶系统的动态动力学拆分,酶促合成手性苯丙氨酸衍生物摘要:通过定向进化得到了来自无色杆菌的突变的α-氨基-ε-己内酰胺(ACL)消旋酶(L19V / L78T),其对苯丙氨酰胺的底物特异性得到了提高。在大肠杆菌(pACLmut / pDBFB40)中共表达的来自拟人O鱼SV3的突变型ACL消旋酶和热稳定的突变型D-氨基酸酰胺酶(DaaA)被用于合成(R)-苯丙氨酸和非天然(R)-苯丙氨酸衍生物(4-OH,4-F,3-F和2-F-Phe)的动力学动力学分辨率(DKR)。重组大肠杆菌(E.coli)与DaaA和突变ACL消旋酶基因催化(合成- [R )-苯丙氨酸与84%产率和99%的ee值由(RS)-苯丙氨酰胺(400 mM)在22小时内。(- [R )-酪氨酸和4-氟- ([R )-苯丙氨酸也被有效地从相应的酰胺化合物的合成。我们还共同expresed从编码突变ACL消旋酶和L-氨基酸酰胺酶的两个基因缺陷短波单胞在大肠杆菌和执行的高效生产各种(的小号)DOI:10.1002/adsc.201100923
-
作为产物:参考文献:名称:Design and bio-evaluation of indole derivatives as potent Kv1.5 inhibitors摘要:Atrial fibrillation (AF) is one of the common arrhythmias that threaten human health. Kv1.5 potassium channel is reported as an efficacious and safe target for the treatment of AF. In this paper, we designed and synthesized three series of compounds through modifying the lead compound RH01617 that was screened out by the pharmacophore model we reported earlier. All of the compounds were evaluated by the whole-patch lamp technology and most of them possessed potent inhibitory activities against Kv1.5. Compounds IIIi and IIIl were evaluated for the target selectivity as well as the pharmacodynamic effects in an isolated rat model. Due to the promising pharmacological behavior, compound IIIl deserves further pharmacodynamic and pharmacokinetic evaluations. (C) 2013 Elsevier Ltd. All rights reserved.DOI:10.1016/j.bmc.2013.08.041
文献信息
-
A Facile Synthesis of N-Substituted Pyrroles作者:Y. Fang、D. Leysen、H. C. J. OttenheijmDOI:10.1080/00397919508015431日期:1995.6Abstract Phosphorous pentoxide is the catalyst of choice for the facile conversion of primary amines, aromatic amines, sulfonamides and primary amides into the corresponding N-substituted pyrroles from 2,5-dimethoxytetrahydrofuran.
-
[EN] PHOSPHONIC ACID DERIVATES AND THEIR USE AS P2Y12 RECEPTOR ANTAGONISTS<br/>[FR] DÉRIVÉS D'ACIDE PHOSPHONIQUE ET LEUR UTILISATION EN TANT QU'ANTAGONISTES DU RÉCEPTEUR P2Y12申请人:ACTELION PHARMACEUTICALS LTD公开号:WO2009069100A1公开(公告)日:2009-06-04The invention relates to 2-phenyl-pyrimidine derivatives containing a phosphonic acid motif and their use as P2Y12 receptor antagonists in the treatment and/or prevention of peripheral vascular, of visceral-, hepatic- and renal-vascular, of cardiovascular and of cerebrovascular diseases or conditions associated with platelet aggregation, including thrombosis in humans and other mammals. (I).
-
14-Membered cyclodepsipeptides with alternating β-hydroxy and α-amino acids by cyclodimerization作者:Boyan Iliev、Anthony Linden、Roland Kunz、Heinz HeimgartnerDOI:10.1016/j.tet.2005.11.002日期:2006.2cyclodimer 10. In all cases, when starting with racemic material, only the trans-substituted cyclodepsipeptides were isolated. Simple molecular modeling revealed that the formation of the cyclodimer is thermodynamically slightly more favorable than that of the cyclomonomer. The proposal that cyclodimer formation is preferred because of the presence of intramolecular H-bonds could not be confirmed by X-ray
-
Controllable Intramolecular Unactivated C(sp3)-H Amination and Oxygenation of Carbamates作者:Qihang Guo、Xiang Ren、Zhan LuDOI:10.1021/acs.orglett.8b03299日期:2019.2.15catalyst-controlled intramolecular unactivated C(sp3)-H amination and oxygenation of carbamates merging visible-light photocatalysis and earth-abundant transition metal catalysis have been reported. Useful amino alcohol and diol derivatives could be selectively obtained from readily available tertiary alcohol derivatives. The possible mechanisms have been proposed via a 1,5-HAT process followed by Lewis acid-controlled
-
Design and Synthesis of Novel Xyloketal Derivatives and Their Protective Activities against H2O2-Induced HUVEC Injury作者:Shixin Liu、Rong Luo、Qi Xiang、Xianfang Xu、Liqin Qiu、Jiyan PangDOI:10.3390/md13020948日期:——In this work, we designed and synthesized a series of amide derivatives (1–13), benzoxazine derivatives (16–28) and amino derivatives (29–30) from xyloketal B. All 28 new derivatives and seven known compounds (14, 15, 31–35) were evaluated for their protection against H2O2-induced HUVEC injury. 23 and 24 exhibited more potential protective activities than other derivatives; and the EC50 values of them and the leading compound 31 (xyloketal B) were 5.10, 3.59 and 15.97 μM, respectively. Meanwhile, a comparative molecular similarity indices analysis (CoMSIA) was constructed to explain the structural activity relationship of these xyloketal derivatives. This 3D QSAR model from CoMSIA suggested that the derived model exhibited good predictive ability in the external test-set validation. Derivative 24 fit well with the COMSIA map, therefore it possessed the highest activity of all compounds. Compounds 23, 24 and 31 (xyloketal B) were further to examine in the JC-1 mitochondrial membrane potential (MMP) assay of HUVECs using flow cytometry (FCM). The result indicated that 23 and 24 significantly inhibited H2O2-induced decrease of the cell mitochondrial membrane potential (ΔΨm) at 25 μM. Collectively, the protective effects of xyloketals on H2O2-induced endothelial cells may be generated from oxidation action by restraining ROS and reducing the MMP.在这项工作中,我们设计并合成了从木梨甲酯B出发的一系列酰胺衍生物(1-13)、苯并恶嗪衍生物(16-28)和氨基衍生物(29-30)。所有28个新衍生物和七个已知化合物(14,15,31-35)都被评估了它们对H2O2诱导的HUVEC损伤的保护作用。23和24表现出比其他衍生物更高的保护活性;它们的EC50值分别为5.10、3.59和15.97μM。同时,我们构建了一个比较分子相似性指数分析(CoMSIA)来解释这些木梨甲酯衍生物的结构活性关系。这个3D QSAR模型来自CoMSIA,表明推导出的模型在外部测试集验证中显示出良好的预测能力。衍生物24很好地符合CoMSIA图,因此它的活性最高。化合物23、24和31(木梨甲酯B)进一步在JC-1线粒体膜电位(MMP)分析中使用流式细胞术(FCM)对HUVECs进行检测。结果表明,23和24在25μM下显著抑制了 诱导的细胞线粒体膜电位(ΔΨm)的降低。总体而言,木梨甲酯对 诱导的血管内皮细胞的保护作用可能来自通过抑制ROS和减少MMP的氧化作用。
表征谱图
-
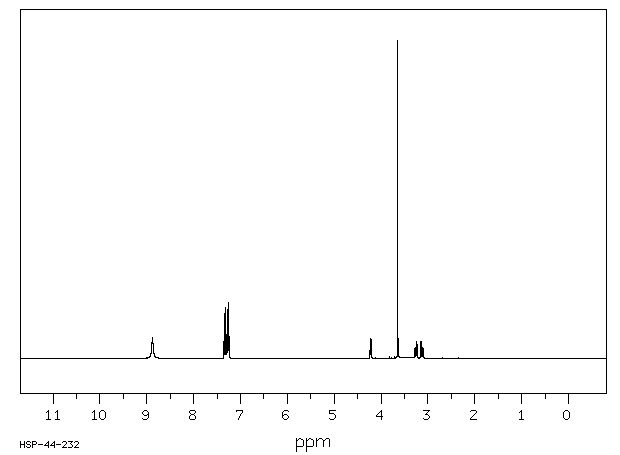
氢谱1HNMR
-
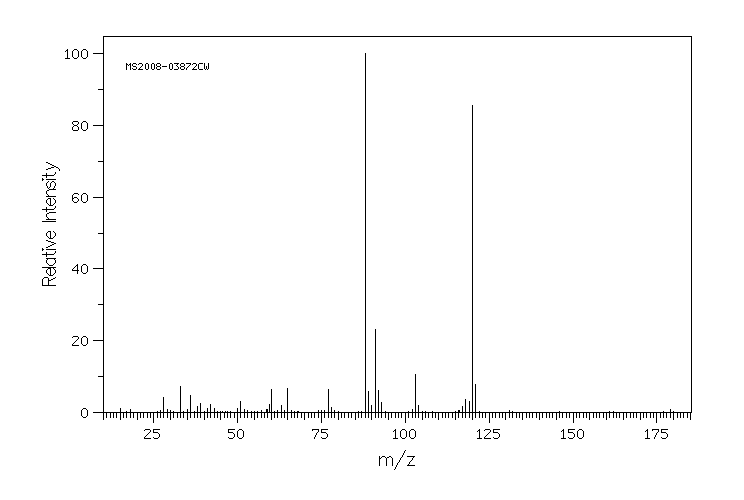
质谱MS
-
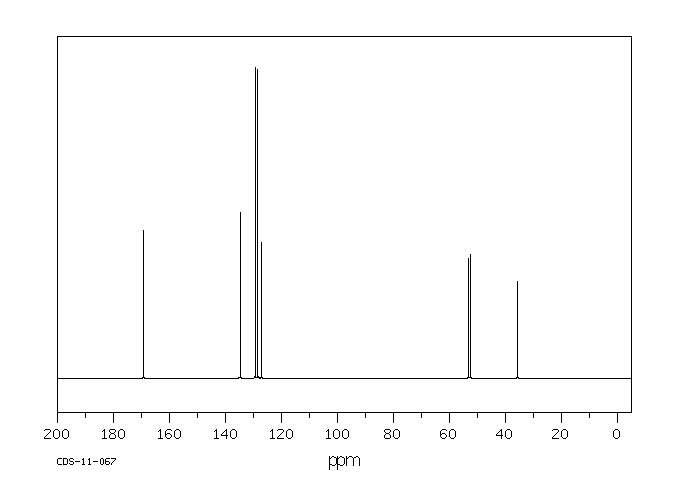
碳谱13CNMR
-
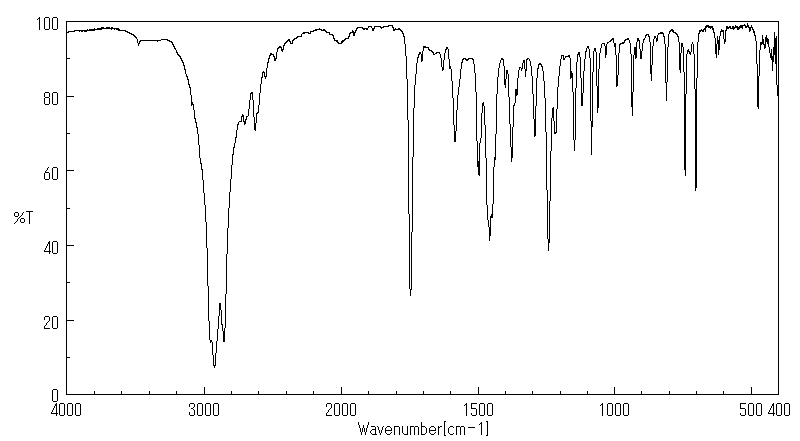
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(甲基3-(二甲基氨基)-2-苯基-2H-azirene-2-羧酸乙酯)
(±)-盐酸氯吡格雷
(±)-丙酰肉碱氯化物
(d(CH2)51,Tyr(Me)2,Arg8)-血管加压素
(S)-(+)-α-氨基-4-羧基-2-甲基苯乙酸
(S)-阿拉考特盐酸盐
(S)-赖诺普利-d5钠
(S)-2-氨基-5-氧代己酸,氢溴酸盐
(S)-2-[[[(1R,2R)-2-[[[3,5-双(叔丁基)-2-羟基苯基]亚甲基]氨基]环己基]硫脲基]-N-苄基-N,3,3-三甲基丁酰胺
(S)-2-[3-[(1R,2R)-2-(二丙基氨基)环己基]硫脲基]-N-异丙基-3,3-二甲基丁酰胺
(S)-1-(4-氨基氧基乙酰胺基苄基)乙二胺四乙酸
(S)-1-[N-[3-苯基-1-[(苯基甲氧基)羰基]丙基]-L-丙氨酰基]-L-脯氨酸
(R)-乙基N-甲酰基-N-(1-苯乙基)甘氨酸
(R)-丙酰肉碱-d3氯化物
(R)-4-N-Cbz-哌嗪-2-甲酸甲酯
(R)-3-氨基-2-苄基丙酸盐酸盐
(R)-1-(3-溴-2-甲基-1-氧丙基)-L-脯氨酸
(N-[(苄氧基)羰基]丙氨酰-N〜5〜-(diaminomethylidene)鸟氨酸)
(6-氯-2-吲哚基甲基)乙酰氨基丙二酸二乙酯
(4R)-N-亚硝基噻唑烷-4-羧酸
(3R)-1-噻-4-氮杂螺[4.4]壬烷-3-羧酸
(3-硝基-1H-1,2,4-三唑-1-基)乙酸乙酯
(2S,4R)-Boc-4-环己基-吡咯烷-2-羧酸
(2S,3S,5S)-2-氨基-3-羟基-1,6-二苯己烷-5-N-氨基甲酰基-L-缬氨酸
(2S,3S)-3-((S)-1-((1-(4-氟苯基)-1H-1,2,3-三唑-4-基)-甲基氨基)-1-氧-3-(噻唑-4-基)丙-2-基氨基甲酰基)-环氧乙烷-2-羧酸
(2S)-2,6-二氨基-N-[4-(5-氟-1,3-苯并噻唑-2-基)-2-甲基苯基]己酰胺二盐酸盐
(2S)-2-氨基-N,3,3-三甲基-N-(苯甲基)丁酰胺
(2S)-2-氨基-3-甲基-N-2-吡啶基丁酰胺
(2S)-2-氨基-3,3-二甲基-N-(苯基甲基)丁酰胺,
(2S)-2-氨基-3,3-二甲基-N-2-吡啶基丁酰胺
(2S,4R)-1-((S)-2-氨基-3,3-二甲基丁酰基)-4-羟基-N-(4-(4-甲基噻唑-5-基)苄基)吡咯烷-2-甲酰胺盐酸盐
(2R,3'S)苯那普利叔丁基酯d5
(2R)-2-氨基-3,3-二甲基-N-(苯甲基)丁酰胺
(2-氯丙烯基)草酰氯
(1S,3S,5S)-2-Boc-2-氮杂双环[3.1.0]己烷-3-羧酸
(1R,5R,6R)-5-(1-乙基丙氧基)-7-氧杂双环[4.1.0]庚-3-烯-3-羧酸乙基酯
(1R,4R,5S,6R)-4-氨基-2-氧杂双环[3.1.0]己烷-4,6-二羧酸
齐特巴坦
齐德巴坦钠盐
齐墩果-12-烯-28-酸,2,3-二羟基-,苯基甲基酯,(2a,3a)-
齐墩果-12-烯-28-酸,2,3-二羟基-,羧基甲基酯,(2a,3b)-(9CI)
黄酮-8-乙酸二甲氨基乙基酯
黄荧菌素
黄体生成激素释放激素(1-6)
黄体生成激素释放激素 (1-5) 酰肼
黄体瑞林
麦醇溶蛋白
麦角硫因
麦芽聚糖六乙酸酯
麦根酸