phlorisobutyrophenone | 35458-21-0
中文名称
——
中文别名
——
英文名称
phlorisobutyrophenone
英文别名
isobutyryl phloroglucinol;2-methyl-1-(2,4,6-trihydroxyphenyl)propan-1-one;2-methyl-1-(2,4,6-trihydroxyphenyl)-1-propanone;1-(2,4,6-trihydroxyphenyl)-2-methylpropanone
CAS
35458-21-0
化学式
C10H12O4
mdl
——
分子量
196.203
InChiKey
BNEBXEZRBLYBCZ-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:138-140 °C
-
沸点:343.9±22.0 °C(Predicted)
-
密度:1.311±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):2.1
-
重原子数:14
-
可旋转键数:2
-
环数:1.0
-
sp3杂化的碳原子比例:0.3
-
拓扑面积:77.8
-
氢给体数:3
-
氢受体数:4
SDS
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 —— robustaol B —— C11H14O4 210.23 —— 1-(2-methylbutanone)-4-O-prenylphloroglucinol 70219-81-7 C15H20O4 264.321 —— 3,5-dihydroxy-4-isobutyrylphenyl acetate 62545-45-3 C12H14O5 238.24 —— 2-Butyryl-4-isopentyl-phloroglucin 22628-86-0 C15H22O4 266.337 —— 1-(4-(E-3,7-dimethylocta-2,6-dienyloxy)-2.6-dihydroxylphenyl)-2-methylpropan-1-one 71539-65-6 C20H28O4 332.44 —— 2-methyl-1-(2,4,6-trihydroxy-3-(3-methylbut-2-en-1-yl)phenyl)propan-1-one 35932-36-6 C15H20O4 264.321 —— 4-Deoxycohumulon 5880-42-2 C20H28O4 332.44 —— 3-geranyl-1-(2'-methylpropanoyl)phloroglucinol —— C20H28O4 332.44 —— 3-<3',3'-dimethylallyl-(1')>-1-isobutyryl-phloroglucinol-6-O-methyl ether 71539-57-6 C16H22O4 278.348 —— 5,7-dihydroxy-6-isobutyryl-2,2-dimethylchromane 35932-38-8 C15H20O4 264.321
反应信息
-
作为反应物:描述:phlorisobutyrophenone 在 sodium hydride 、 对甲苯磺酸 作用下, 以 四氢呋喃 、 苯 为溶剂, 反应 6.66h, 生成 6,8-dihydroxy-7-(1-(2-hydroxy-3,3,5,5-tetramethyl-4,6-dioxocyclohex-1-enyl)-2-methylpropyl)-5-isobutyryl-9-isopropyl-2,2,4,4-tetramethyl-2H-xanthene-1,3(4H,9H)-dione参考文献:名称:靶向mPGES-1和5-脂氧合酶的新型Myttucommulones和结构类似物的合成和生物学评估摘要:天然酰基间苯三酚Myrtucommulone A(1)抑制微粒体前列腺素E 2合酶(mPGES)-1和5-脂氧合酶(5-LO),并诱导癌细胞凋亡。从1个铅开始,按照简单的模块化策略合成了28个类似物,并产生了高收率的收敛步骤。主要的结构变化涉及(I)用二甲基二酮或茚满二酮取代同型二羧酸部分,(II)用酰基间苯三酚核心将同型二羧酸环化,以及(III)用异丙基,异丁基,n取代次甲基桥和酰基残基-戊基或苯基。抑制mPGES-1的效力提高了12.5倍,达到43(2-(1-(3-己基-2,4,6-三羟基-5-(1-(3-羟基-1-氧代-1H-茚满-2-基)-2-甲基丙基)苯基)-2 -甲基丙基)-3-羟基-1H-茚满-1-酮,IC 50 = 0.08μM,5-LO抑制作用被47(2-((3-hexanoyl-2,4,6-具有IC 50的三羟基-5-((3-羟基-1-氧代-1H-茚满-2-基)(苯基DOI:10.1016/j.ejmech.2015.06.001
-
作为产物:描述:1-[(2-methylpropanoyl)phloroglucinyl]-β-D-glucopyranoside 在 盐酸 作用下, 反应 0.5h, 以28 mg的产率得到phlorisobutyrophenone参考文献:名称:Anti-inflammatory Acylphloroglucinol Derivatives from Hops (Humulus lupulus)摘要:The polyphenol-enriched fraction of an ethanolic hops extract (Humulus lupulus) was separated to provide four acylphloroglucinol-glucopyranosides (1-4). 1-(2-Methylpropanoyl)phloroglucinol-glucopyranoside 1 has been isolated from hops before, whereas 1-(2-methylbutyryl)phloroglucinol-glucopyranoside 2, known as multifidol glucoside, and 1-(3-methylbutyryl)phloroglucinol-glucopyranoside 3 were found in hops for the first time. 5-(2-Methylpropanoyl)phloroglucinol-glueopyranoside 4 was identified as a new natural product. The compounds were tested for inhibition of COX-1 activity. The aglycon 5, obtained by acid hydrolysis of 1, was equally effective as phloroglucinol, with an IC50 of 3.8 mu M. The inhibitory potential of the glucosides was 1 > 2 > 3 and decreased with increasing length of the acyl side chain. Compound 4 was about 2.5-fold less active than 1 (IC50: 23.7 and 58.7 mu M, respectively).DOI:10.1021/np050164z
-
作为试剂:描述:水芹烯 、 2,3-二氯-5,6-二氰基-1,4-苯醌 在 iron(III) chloride 、 phlorisobutyrophenone 作用下, 以 N,N-二甲基甲酰胺 为溶剂, 反应 4.0h, 以57%的产率得到(4aR,8aS)-6,7-dichloro-9-isopropyl-2-methyl-5,8-dioxo-1,4,5,8-tetrahydro-1,4-ethanonaphthalene-4a,8a-dicarbonitrile参考文献:名称:通过氧化[3 + 2]环加成法仿生合成Callistrilones A–E摘要:可以从7种和市售的α-水芹烯(8-)中获得准确的Callistrilones A-E合成。合成策略主要是受生物遗传学假设的启发,是通过氧化[3 + 2]环加成反应,随后的迈克尔加成反应和分子内亲核加成反应来构建目标分子的。此外,还合成了Viminalin I,并明确证实了其绝对构型。DOI:10.1021/acs.orglett.8b00238
文献信息
-
Rapid Synthesis of Polyprenylated Acylphloroglucinol Analogs via Dearomative Conjunctive Allylic Annulation作者:Alexander J. Grenning、Jonathan H. Boyce、John A. PorcoDOI:10.1021/ja5060302日期:2014.8.20Polyprenylated acylphloroglucinols (PPAPs) are structurally complex natural products with promising biological activities. Herein, we present a biosynthesis-inspired, diversity-oriented synthesis approach for rapid construction of PPAP analogs via double decarboxylative allylation (DcA) of acylphloroglucinol scaffolds to access allyl-desoxyhumulones followed by dearomative conjunctive allylic alkylation
-
[EN] PROCESSES FOR THE PREPARATION OF ORTHO-ALLYLATED HYDROXY ARYL COMPOUNDS<br/>[FR] PROCÉDÉS DE PRÉPARATION DE COMPOSÉS HYDROXY-ARYLE ORTHO-ALLYLÉS申请人:UNIV MCMASTER公开号:WO2021237371A1公开(公告)日:2021-12-02The present application describes process for preparing an ortho-allylated hydroxy aryl compounds such as compounds of Formula (I) by reacting an allylic alcohol with a hydroxy aryl compound in the presence of aluminum compound selected from alumina and aluminum alkoxides and in a non-protic solvent wherein at least one carbon atom ortho to the hydroxy group in the hydroxy aryl compound is unsubstituted. The present application also includes compounds of Formula (I).
-
Inhibitory Effect of Acylphloroglucinol Derivatives on the Replication of Vesicular Stomatitis Virus作者:Kazuhiro Chiba、Takako Takakuwa、Masahiro Tada、Takao YoshiiDOI:10.1271/bbb.56.1769日期:1992.1The antiviral activity of natural phloroglucinols and of synthesized mono- and diacylphloroglucinols, and 2,6-diacyl-4,4-dialkylcyclohexa-1,3,5-triones was investigated. A correlation between the acyl chain length and inhibitory activity against vesicular stomatitis virus (VSV) was observed. Potent antiviral activity was found in di-isovalerylphoroglucinol. 2,6-Diacyl-4,4-dialkylcyclohexa-1,3,5-triones inhibited replication of the virus with low cytotoxicity.
-
Synthesis of mammeins and surangin a作者:Leslie Crombie、Raymond C.F. Jones、Christopher J. PalmerDOI:10.1016/s0040-4039(00)98874-9日期:1985.1Syntheses of natural 4-alkyl and 4-aryl coumarins with hexasubstituted aromatic rings, uncouplers of oxidative phosphorylation, are reported. Mammea B/BB, by synthesis, is the (S)-(−)-compound.
-
Isolation, Structure Elucidation, and Total Synthesis of Myrtuspirone A from <i>Myrtus communis</i>作者:Min-Jing Cheng、Xin-Yi Yang、Jia-Qing Cao、Chao Liu、Li-Ping Zhong、Ying Wang、Xue-Fu You、Chuang-Chuang Li、Lei Wang、Wen-Cai YeDOI:10.1021/acs.orglett.9b00108日期:2019.3.15A pair of enantiomeric triketone–phloroglucinol hybrids, (+)- and (−)-myrtuspirone A (1), featuring an unprecedented 3-isopropyl-3H-spiro[benzofuran-2,1′-cyclohexane] backbone, were isolated from the leaves of Myrtus communis. The absolute configuration of each enantiomer of 1 was determined by X-ray diffraction and chemical calculations. Furthermore, the gram-scale total syntheses of (±)-1 and (−)-1
表征谱图
-
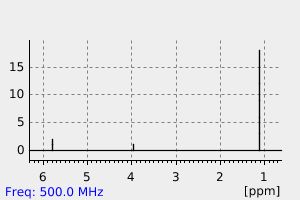
氢谱1HNMR
-
质谱MS
-
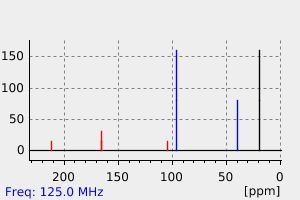
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(反式)-4-壬烯醛
(s)-2,3-二羟基丙酸甲酯
([1-(甲氧基甲基)-1H-1,2,4-三唑-5-基](苯基)甲酮)
(Z)-4-辛烯醛
(S)-氨基甲酸酯β-D-O-葡糖醛酸
(S)-3-(((2,2-二氟-1-羟基-7-(甲基磺酰基)-2,3-二氢-1H-茚满-4-基)氧基)-5-氟苄腈
(R)-氨基甲酸酯β-D-O-葡糖醛酸
(5,5-二甲基-2-(哌啶-2-基)环己烷-1,3-二酮)
(2,5-二氟苯基)-4-哌啶基-甲酮
龙胆苦苷
龙胆二糖甲乙酮氰醇(P)
龙胆二糖丙酮氰醇(P)
龙胆三糖
龙涎酮
齐罗硅酮
齐留通beta-D-葡糖苷酸
鼠李糖
黑芥子苷单钾盐
黑海棉酸钠盐
黑木金合欢素
黑曲霉三糖
黑介子苷
黄尿酸8-O-葡糖苷
麻西那霉素II
麦迪霉素
麦芽糖脎
麦芽糖基海藻糖
麦芽糖1-磷酸酯
麦芽糖
麦芽四糖醇
麦芽四糖
麦芽十糖
麦芽六糖
麦芽五糖水合物
麦芽五糖
麦芽五糖
麦芽五糖
麦芽三糖醇
麦芽三糖
麦芽三糖
麦芽三塘水合
麦芽七糖水合物
麦芽七糖
麦法朵
麦可酚酸-酰基-Β-D-葡糖苷酸
麦利查咪
麝香酮
鹤草酚
鸢尾酚酮 3-C-beta-D-吡喃葡萄糖苷
鸡矢藤苷