(+/-)-menthol | 499-69-4
中文名称
——
中文别名
——
英文名称
(+/-)-menthol
英文别名
Carvomenthol;p-menthan-2-ol;Carvacrol;2-methyl-5-propan-2-ylcyclohexan-1-ol
CAS
499-69-4;3858-45-5;3127-80-8;1845-59-6;60320-28-7;5563-78-0
化学式
C10H20O
mdl
——
分子量
156.268
InChiKey
ULJXKUJMXIVDOY-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
沸点:217-218 °C
-
密度:0.908 g/cm3
-
LogP:3.20
-
折光率:1.462-1.463
-
保留指数:1205
计算性质
-
辛醇/水分配系数(LogP):3
-
重原子数:11
-
可旋转键数:1
-
环数:1.0
-
sp3杂化的碳原子比例:1.0
-
拓扑面积:20.2
-
氢给体数:1
-
氢受体数:1
SDS
制备方法与用途
毒性
GRAS(FEMA)。
使用限量- 无醇饮料:2.2 mg/kg
- 喇叭醇饮料:4.0 mg/kg
- 凝胶制品和布丁类:4.1 mg/kg
- 冷冻乳品:7.6 mg/kg
- 焙烤制品、软糖:14.8 mg/kg
上下游信息
-
下游产品
中文名称 英文名称 CAS号 化学式 分子量 (2-甲基-5-丙-2-基-环己基)乙酸酯 2-methyl-5-(propan-2-yl)cyclohexyl acetate 7460-78-8 C12H22O2 198.305
反应信息
-
作为反应物:描述:参考文献:名称:烷基硼化合物与儿茶酚的自由基链还原摘要:烷基硼烷向相应烷烃的转化通常通过烷基硼烷的质子分解进行。这个简单的反应需要使用严格的反应条件,即在高温(> 150 °C)下用羧酸处理。我们在这里报告了一种将有机硼烷转化为烷烃的温和激进程序。4-叔丁基儿茶酚是一种成熟的自由基抑制剂和抗氧化剂,是氢原子的来源。由于苯氧基自由基对烷基硼烷的特殊反应性,观察到有效的链式反应。该反应已应用于广泛的有机硼衍生物,例如 B-烷基儿茶酚硼烷、三烷基硼烷、频哪醇硼酸酯和烷基硼酸。此外,迄今为止,通过实验确定了仲烷基自由基和邻苯二酚衍生物之间的氢转移的难以捉摸的速率常数。有趣的是,它们在 80 °C 时比氢化锡慢不到 1 个数量级,这使得儿茶酚对涉及 CC 键形成的广泛转化特别有吸引力。DOI:10.1021/ja110224d
-
作为产物:参考文献:名称:Antimicrobial therapeutic compositions for oral and topical use摘要:该发明提供了含有天然酚类化合物和精油醇的治疗抗菌组合物,以及使用方法。这些治疗抗菌组合物可用于治疗内部和外部的细菌、真菌和原虫感染,以及抗生素耐药和次级的机会性感染。公开号:US20030225003A1
文献信息
-
Reagents and synthetic methods—40作者:J.M. Aizpurua、M. Juaristi、B. Lecea、C. PalomoDOI:10.1016/s0040-4020(01)96614-1日期:1985.1efficient for the oxidation of alcohols to carbonyl compounds, for the oxidative coupling of mercaptans into disulfides and for a mild cleavage of oximes to carbonyl compounds. Chlorotrimethylsilane-chromium trioxide has been shown to be an efficient oxidizing agent for the conversion of arylmethanes to benzaldehydes. The reagent is applied to the oxidative cleavage of some benzyl esters. A mild procedure
-
A new ferrocene-based bulky pyridine as an efficient reusable homogeneous catalyst作者:Bishwapran Kashyap、Prodeep PhukanDOI:10.1039/c3ra41674g日期:——An effective approach to reusing a homogeneous catalyst has been demonstrated. A ferrocene-based bulky pyridine has been synthesized and utilized as a homogeneous catalyst for the synthesis of benzoylfumarates as well as for acetylation. After the reaction, the catalyst was separated by simple precipitation and reused without appreciable loss of activity.
-
A mild and efficient method for esterification and transesterification catalyzed by iodine作者:K Ramalinga、P Vijayalakshmi、T.N.B KaimalDOI:10.1016/s0040-4039(01)02235-3日期:2002.1a practical and useful Lewis acid catalyst for the esterification of carboxylic acids with alcohols. The high catalytic activity of iodine can be used for the transesterification of esters by different alcohols including tertiary alcohols and sterically hindered primary and secondary alcohols. The method presented is especially effective for simultaneous esterification and transesterification reactions
-
PROTECTION OF ALCOHOLS AND ACIDS WITH ALLYLSILANES CATALYZED BY IODINE OR IODOTRIMETHYLSILANE IN CHLORINATED HYDROCARBON作者:Akira Hosomi、Hideki SakuraiDOI:10.1246/cl.1981.85日期:1981.1.5Many triorganosilyl ethers and esters were prepared by the reaction of allylsilanes with alcohols catalyzed by iodine or iodotrimethylsilane in excellent yields. Bromine and bromotrimethylsilane were also effective catalysts.
-
An alternative to the Swern oxidation作者:Alakesh Bisai、M. Chandrasekhar、Vinod K. SinghDOI:10.1016/s0040-4039(02)02004-x日期:2002.11A variety of alcohols have been oxidized under mild conditions by the DMSO–Ph3P·X2 complexes. The reaction does not produce any Pummerer product. A mechanism for the reaction is proposed.DMSO–Ph 3 P·X 2络合物在温和条件下已氧化了多种醇。该反应不产生任何Pummerer产物。提出了反应机理。
表征谱图
-
氢谱1HNMR
-
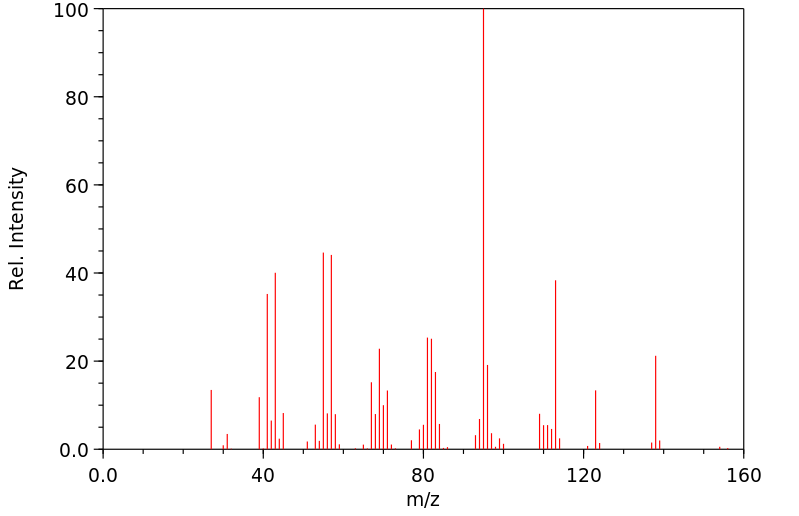
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(5β,6α,8α,10α,13α)-6-羟基-15-氧代黄-9(11),16-二烯-18-油酸
(3S,3aR,8aR)-3,8a-二羟基-5-异丙基-3,8-二甲基-2,3,3a,4,5,8a-六氢-1H-天青-6-酮
(2Z)-2-(羟甲基)丁-2-烯酸乙酯
(2S,4aR,6aR,7R,9S,10aS,10bR)-甲基9-(苯甲酰氧基)-2-(呋喃-3-基)-十二烷基-6a,10b-二甲基-4,10-dioxo-1H-苯并[f]异亚甲基-7-羧酸盐
(1aR,4E,7aS,8R,10aS,10bS)-8-[((二甲基氨基)甲基]-2,3,6,7,7a,8,10a,10b-八氢-1a,5-二甲基-氧杂壬酸[9,10]环癸[1,2-b]呋喃-9(1aH)-酮
(+)顺式,反式-脱落酸-d6
龙舌兰皂苷乙酯
龙脑香醇酮
龙脑烯醛
龙脑7-O-[Β-D-呋喃芹菜糖基-(1→6)]-Β-D-吡喃葡萄糖苷
龙牙楤木皂甙VII
龙吉甙元
齿孔醇
齐墩果醛
齐墩果酸苄酯
齐墩果酸甲酯
齐墩果酸溴乙酯
齐墩果酸二甲胺基乙酯
齐墩果酸乙酯
齐墩果酸3-O-alpha-L-吡喃鼠李糖基(1-3)-beta-D-吡喃木糖基(1-3)-alpha-L-吡喃鼠李糖基(1-2)-alpha-L-阿拉伯糖吡喃糖苷
齐墩果酸 beta-D-葡萄糖酯
齐墩果酸 beta-D-吡喃葡萄糖基酯
齐墩果酸 3-乙酸酯
齐墩果酸 3-O-beta-D-葡吡喃糖基 (1→2)-alpha-L-吡喃阿拉伯糖苷
齐墩果酸
齐墩果-12-烯-3b,6b-二醇
齐墩果-12-烯-3,24-二醇
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,21,23-三醇,(3b,4b,21a)-(9CI)
齐墩果-12-烯-3,11-二酮
齐墩果-12-烯-2α,3β,28-三醇
齐墩果-12-烯-29-酸,3,22-二羟基-11-羰基-,g-内酯,(3b,20b,22b)-
齐墩果-12-烯-28-酸,3-[(6-脱氧-4-O-b-D-吡喃木糖基-a-L-吡喃鼠李糖基)氧代]-,(3b)-(9CI)
齐墩果-12-烯-28-酸,3,7-二羰基-(9CI)
齐墩果-12-烯-28-酸,3,21,29-三羟基-,g-内酯,(3b,20b,21b)-(9CI)
鼠特灵
鼠尾草酸醌
鼠尾草酸
鼠尾草酚酮
鼠尾草苦内脂
黑蚁素
黑蔓醇酯B
黑蔓醇酯A
黑蔓酮酯D
黑海常春藤皂苷A1
黑檀醇
黑果茜草萜 B
黑五味子酸
黏黴酮
黏帚霉酸