2-(溴乙酰基)-6-甲氧基萘 | 10262-65-4
中文名称
2-(溴乙酰基)-6-甲氧基萘
中文别名
2-(2-溴乙酰基)-6-甲氧基萘
英文名称
2-bromo-1-(6-methoxy-2-naphthyl)ethanone
英文别名
2-bromo-6'-methoxy-2'-acetonaphthone;2-bromoacetyl-6-methoxynaphthalene;2-Bromacetyl-6-methoxy-naphthalin;2-(Bromoacetyl)-6-methoxynaphthalene;2-bromo-1-(6-methoxynaphthalen-2-yl)ethanone
CAS
10262-65-4
化学式
C13H11BrO2
mdl
MFCD03964189
分子量
279.133
InChiKey
HHHKEQGAGUAOQI-UHFFFAOYSA-N
BEILSTEIN
——
EINECS
——
-
物化性质
-
计算性质
-
ADMET
-
安全信息
-
SDS
-
制备方法与用途
-
上下游信息
-
文献信息
-
表征谱图
-
同类化合物
-
相关功能分类
-
相关结构分类
物化性质
-
熔点:110.0 to 114.0 °C
-
沸点:387.2±17.0 °C(Predicted)
-
密度:1.451±0.06 g/cm3(Predicted)
计算性质
-
辛醇/水分配系数(LogP):3.6
-
重原子数:16
-
可旋转键数:3
-
环数:2.0
-
sp3杂化的碳原子比例:0.153
-
拓扑面积:26.3
-
氢给体数:0
-
氢受体数:2
安全信息
-
危险等级:8
-
危险品运输编号:3261
-
海关编码:2914700090
-
包装等级:II
-
危险类别:8
-
危险性防范说明:P234,P260,P264,P280,P301+P330+P331+P310,P303+P361+P353+P310+P363,P304+P340+P310,P305+P351+P338+P310,P390,P405,P406,P501
-
危险性描述:H290,H314
-
储存条件:室温且干燥
SDS
2-(Bromoacetyl)-6-methoxynaphthalene Revision number: 1
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 2-(Bromoacetyl)-6-methoxynaphthalene
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
2-(Bromoacetyl)-6-methoxynaphthalene
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component(s): 2-(Bromoacetyl)-6-methoxynaphthalene
Percent: >98.0%(GC)
CAS Number: 10262-65-4
Synonyms: 2-Bromo-6'-methoxy-2'-acetonaphthone
Chemical Formula: C13H11BrO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
2-(Bromoacetyl)-6-methoxynaphthalene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:112 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents, strong bases
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
2-(Bromoacetyl)-6-methoxynaphthalene
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3261
Proper shipping name: Corrosive solid, acidic, organic, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
SAFETY DATA SHEET
Section 1. BASE INFORMATION
Product name: 2-(Bromoacetyl)-6-methoxynaphthalene
Revision number: 1
Section 2. HAZARDS IDENTIFICATION
Classification of the GHS
PHYSICAL HAZARDS
Corrosive to metals Category 1
HEALTH HAZARDS
Skin corrosion/irritation Category 1B
Category 1
Serious eye damage/eye irritation
ENVIRONMENTAL HAZARDS Not classified
GHS label elements
Pictograms or hazard symbols
Signal word Danger
Hazard statement May be corrosive to metals
Causes severe skin burns and eye damage
Precautionary statements
Keep only in original container.
[Prevention]
Do not breathe.
Wash hands thoroughly after handling.
Wear protective gloves/eye protection/face protection.
IF INHALED: Remove victim to fresh air and keep at rest in a position comfortable for
[Response]
breathing.
IF SWALLOWED: Rinse mouth. Do NOT induce vomiting.
IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses,
if present and easy to do. Continue rinsing.
IF ON SKIN (or hair): Remove/Take off immediately all contaminated clothing. Rinse
skin with water/shower.
Wash contaminated clothing before reuse.
Immediately call a POISON CENTER or doctor/physician.
Absorb spillage to prevent material damage.
[Storage] Store locked up.
[Disposal] Dispose of contents/container through a waste management company authorized by
the local government
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Substance
Substance/mixture:
2-(Bromoacetyl)-6-methoxynaphthalene
Section 3. COMPOSITION/INFORMATION ON INGREDIENTS
Component(s): 2-(Bromoacetyl)-6-methoxynaphthalene
Percent: >98.0%(GC)
CAS Number: 10262-65-4
Synonyms: 2-Bromo-6'-methoxy-2'-acetonaphthone
Chemical Formula: C13H11BrO2
Section 4. FIRST AID MEASURES
Inhalation: Remove victim to fresh air and keep at rest in a position comfortable for breathing.
Immediately call a POISON CENTER or doctor/physician.
Skin contact: Remove/Take off immediately all contaminated clothing. Gently wash with plenty of
soap and water. Immediately call a POISON CENTER or doctor/physician.
Eye contact: Rinse cautiously with water for several minutes. Remove contact lenses, if present
and easy to do. Continue rinsing.Immediately call a POISON CENTER or
doctor/physician.
Ingestion: Immediately call a POISON CENTER or doctor/physician. Rinse mouth. Do NOT
induce vomiting.
Protection of first-aiders: A rescuer should wear personal protective equipment, such as rubber gloves and air-
tight goggles.
Section 5. FIRE-FIGHTING MEASURES
Suitable extinguishing Dry chemical, foam, water spray, carbon dioxide.
media:
Specific hazards: Take care as it may decompose upon combustion or in high temperatures to
generate poisonous fume.
Specific methods: Fire-extinguishing work is done from the windward and the suitable fire-extinguishing
method according to the surrounding situation is used. Uninvolved persons should
evacuate to a safe place. In case of fire in the surroundings: Remove movable
containers if safe to do so.
Special protective When extinguishing fire, be sure to wear personal protective equipment.
equipment for firefighters:
Section 6. ACCIDENTAL RELEASE MEASURES
Use extra personal protective equipment (P3 filter respirator for toxic particles). Keep
Personal precautions,
protective equipment and people away from and upwind of spill/leak. Entry to non-involved personnel should
emergency procedures: be controlled around the leakage area by roping off, etc.
Environmental precautions: Prevent product from entering drains.
Methods and materials for Sweep dust to collect it into an airtight container, taking care not to disperse it.
containment and cleaning Adhered or collected material should be promptly disposed of, in accordance with
up: appropriate laws and regulations.
Section 7. HANDLING AND STORAGE
Handling
Technical measures: Handling is performed in a well ventilated place. Wear suitable protective equipment.
Prevent dispersion of dust. Wash hands and face thoroughly after handling.
Use a closed system if possible. Use a local exhaust if dust or aerosol will be
generated.
Advice on safe handling: Avoid contact with skin, eyes and clothing.
Use corrosive resistant equipment.
Storage
Storage conditions: Keep container tightly closed. Store in a cool and dark place.
Store locked up.
Store away from incompatible materials such as oxidizing agents.
Packaging material: Law is followed. Keep only in original container.
2-(Bromoacetyl)-6-methoxynaphthalene
Section 8. EXPOSURE CONTROLS / PERSONAL PROTECTION
Engineering controls: Install a closed system or local exhaust. Also install safety shower and eye bath.
Personal protective equipment
Respiratory protection: Dust respirator, self-contained breathing apparatus(SCBA), supplied air respirator,
etc. Use respirators approved under appropriate government standards and follow
local and national regulations.
Impervious gloves.
Hand protection:
Eye protection: Safety goggles. A face-shield, if the situation requires.
Skin and body protection: Impervious protective clothing. Protective boots, if the situation requires.
Section 9. PHYSICAL AND CHEMICAL PROPERTIES
Physical state (20°C): Solid
Form: crystal - powder
White - Pale yellow
Color:
Odor: No data available
pH: No data available
Melting point/freezing point:112 °C
Boiling Point/Range: No data available
Flash Point: No data available
Explosive limits
Lower: No data available
No data available
Upper:
Density: No data available
No data available
Solubility:
Section 10. STABILITY AND REACTIVITY
Stability: Stable under proper conditions.
Reactivity: No special reactivity has been reported.
Incompartible materials: oxidizing agents, strong bases
Hazardous Decomposition Carbon monoxide, Carbon dioxide, Hydrogen bromide
Products:
Section 11. TOXICOLOGICAL INFORMATION
Acute Toxicity: No data available
Skin corrosion/irritation: No data available
Serious eye No data available
damage/irritation:
Germ cell mutagenicity: No data available
Carcinogenicity:
IARC = No data available
No data available
NTP =
Reproductive toxicity: No data available
Section 12. ECOLOGICAL INFORMATION
Ecotoxicity:
Fish: No data available
Crustacea: No data available
Algae: No data available
Persistence / degradability: No data available
Bioaccumulative No data available
potential(BCF):
Mobillity in soil
log Pow: No data available
Soil adsorption (Koc): No data available
Henry's Law No data available
constant(PaM3/mol):
2-(Bromoacetyl)-6-methoxynaphthalene
Section 13. DISPOSAL CONSIDERATIONS
Recycle to process, if possible. Consult your local regional authorities. You may be able to dissolve or mix material
with a combustible solvent and burn in a chemical incinerator equipped with an afterburner and scrubber system.
Observe all federal, state and local regulations when disposing of the substance.
Section 14. TRANSPORT INFORMATION
Hazards Class: 8: Corrosive.
UN-No: 3261
Proper shipping name: Corrosive solid, acidic, organic, n.o.s.
II
Packing group:
Section 15. REGULATORY INFORMATION
Safe management ordinance of dangerous chemical product (State Council announces on January 26,
2002): Safe use and production, the storage of a dangerous chemical, transport, loading and unloading were
prescribed.
SECTION 16 - ADDITIONAL INFORMATION
N/A
制备方法与用途
磁性金属配合物
上下游信息
-
上游原料
中文名称 英文名称 CAS号 化学式 分子量 6-甲氧基-2-乙酰萘 6-methoxy-2-acetylnaphthalene 3900-45-6 C13H12O2 200.237 -
下游产品
中文名称 英文名称 CAS号 化学式 分子量 2,2-二溴-1-(6-甲氧基萘-2-基)乙酮 α,α'-dibromoacetyl-2 methoxy-6 naphtalene 52997-56-5 C13H10Br2O2 358.029 —— 2-bromo-1-(6-hydroxy-2-naphthyl)ethanone 1315483-07-8 C12H9BrO2 265.106 —— 2-(aminoacetyl)-6-methoxynaphthalene 792879-88-0 C13H13NO2 215.252 —— 2-azido-1-(6-methoxynaphthalen-2-yl)ethanone 1001422-37-2 C13H11N3O2 241.249 —— (6-Methoxy-2-naphthacyl)-benzoat 62244-94-4 C20H16O4 320.345 —— 2-Imidazol-1-yl-1-(6-methoxynaphthalen-2-yl)ethanone —— C16H14N2O2 266.299 —— 2-(6-methoxy-2-naphthyl)ethynyl methyl sulfide 1391948-70-1 C14H12OS 228.315 —— 2-(6-methoxy-2-naphthyl)ethynyl ethyl sulfide 1391948-81-4 C15H14OS 242.342 —— bis[2-(6-methoxy-2-naphthyl)ethynyl] sulfide 1391976-33-2 C26H18O2S 394.494
反应信息
-
作为反应物:描述:参考文献:名称:吲哚美辛的2-(2-芳基吗啉代-4-基)乙基酯作为潜在的环氧合酶2(COX-2)抑制剂的设计,合成和生物学评估摘要:合成了许多新型的2-(2-芳基吗啉代-4-基)乙基1-(4-氯苯甲酰基)-5-甲氧基-2-甲基-1 H-吲哚-3-乙酸盐酸盐,并测试了其环氧合酶(COX ‐1和COX‐2)体外抑制特性。这些化合物中的许多化合物都表现出中等至良好的选择性COX-2抑制作用,并且酯部分侧链上取代基的细微结构变化显着改变了抑制性能。2- [2-(4-丁氧基苯基)吗啉代-4-基]乙基1-(4-氯苯甲酰基)-5-甲氧基-2-甲基-1 H-吲哚-3-乙酸盐酸盐(1f)具有良好的选择性COX-2抑制活性(选择性指数(SI)182),与二芳基吡唑的COX-2抑制剂塞来昔布(SI 214)比较。而2- [2-(2-(2,4-二氯-5-氟苯基)吗啉代-4-基]乙基1-(4-氯苯甲酰基)-5-甲氧基-2-甲基-1 H-吲哚-3-乙酸盐酸盐(1g)比塞来昔布具有更高的选择性COX-2抑制活性(SI 358)。两种化合物均被认为是DOI:10.1002/cjoc.201200196
-
作为产物:描述:参考文献:名称:使用N-溴代琥珀酰亚胺对酮进行光化学α-溴化:一种简单,温和且有效的方法摘要:芳香族和脂肪族羰基化合物在N-溴琥珀酰亚胺的紫外可见光下易溴化,从而以高收率在低温(30°C)下得到相应的α-溴代酮,而无需任何催化剂,催化剂载体或自由基引发剂,并且在短时间。DOI:10.1016/j.tetlet.2006.12.101
文献信息
-
2,3-Diaroyl benzofurans from arynes: sequential synthesis of 2-aroyl benzofurans followed by benzoylation作者:Kashmiri Neog、Babulal Das、Pranjal GogoiDOI:10.1039/c8ob00631h日期:——strategy for the direct synthesis of 2-aroyl benzofurans from aryne precursors has been developed. This reaction proceeds via C–O and C–C bond cleavage as well as C–O and C–C bond formation in a single reaction vessel. The methodology provides good yields of 2-aroyl benzofurans and tolerates a variety of functional groups. The synthesized 2-aroyl benzofurans were further benzoylated at 3-positions and
-
FACILE SYNTHESIS OF BIOLOGICALLY ACTIVE LINEAR BISAROYL BENZODIFURANS BY PTC AND SOLVENT FREE MICROWAVE IRRADIATION作者:K. S. Krishna Murthy、B. Rajitha、M. Kanakalingeswara Rao、T. Raja Komuraiah、S. M. ReddyDOI:10.1515/hc.2002.8.2.179日期:2002.1(Or) (i i) K 2 C O J / M W 3 0 0 / 1 3 min -X Ar 4-H C6H4 4-CI C6H4 4-Ph C6H4 2-OMe O C T 4-NO, C6H, 4-Me C6H, 4-OMe C6H, 3 a-e 4 a-g 5 a-g [R=CHJ] [R=C2H.J [R=C6H5] Scheme 1 The benzodifurans were characterized by UV, IR, NMR and Mass spectral data (Table 1). The UV spectra of all the compounds have displayed two absorption bands in the regions λTM, 322327 and 355-359 nm as compared with unsubstituted2,6-二芳酰基/萘酰基-3,5-二烷基/苯基-苯并[l,2-b;5,4-b']二呋喃(3a-e、4a-g和5a-g)已通过缩合合成4,6-二酰基/二芳酰基间苯二酚 (1 i-iii) 与各种 p-取代的 a 溴酮 (2a-g) 通过 (a) 相转移催化方法和 (b) 微波辐射。对这两种方法进行了比较。已发现微波辐射是合成双芳酰基苯并二呋喃的有效途径。所有化合物都经过了抗菌筛选(3a-e、4a-g 和 5b-g),一些选定的化合物已经过抗植入活性测试。苯并二呋喃 3c、3d、3e、4a、4b、4d、4f 和 5g 对革兰氏阳性菌显示出优异的活性,化合物 4c 在 10 mg/Kg/大鼠/天对白化大鼠显示出 67% 的抗植入活性。介绍 已知天然和合成的苯并呋喃衍生物与生物和药理活性有关。Methyl-2-methyl-5-hydroxy-6-acetyl-benzofuran-3carboxylate
-
Usnic Acid Enaminone-Coupled 1,2,3-Triazoles as Antibacterial and Antitubercular Agents作者:Pavan K. Bangalore、Siva K. Vagolu、Rakesh K. Bollikanda、Dileep K. Veeragoni、Pallavi C. Choudante、Sunil Misra、Dharmarajan Sriram、Balasubramanian Sridhar、Srinivas KantevariDOI:10.1021/acs.jnatprod.9b00475日期:2020.1.24be the most active analogue, inhibiting Mycobacterium tuberculosis (Mtb) at an MIC value of 2.5 μM. Analogues 16 and 27, with 3,4-difluorophenacyl and 2-acylnaphthalene units, respectively, inhibited Mtb at MIC values of 5.4 and 5.3 μM, respectively. Among the tested Gram-positive and Gram-negative bacteria, the new derivatives were active on Bacillus subtilis, with compounds 18 [3-(trifluoromethyl)phenacyl](+)-尿酸是地衣中的二次代谢产物,具有广泛的生物学特性,例如抗肿瘤,抗微生物,抗病毒,抗炎和杀虫活性。对这些药理活性感兴趣并挖掘其潜力,我们在此介绍新的松萝酸烯胺酮偶联的1,2,3-三唑10-44作为抗分枝杆菌药的合成和生物学评估。将(+)-松香酸与炔丙基胺缩合,得到具有末端乙炔基部分的松香酸烯胺酮8。在铜催化下,它进一步与各种叠氮化物A1-A35反应,以高收率得到三唑10-44。在合成的化合物中,糖精衍生物36被证明是活性最高的类似物,在MIC值为2.5μM时可抑制结核分枝杆菌(Mtb)。类似物16和27,以及3,4-二氟苯酰基和2-酰基萘单元分别在MIC值为5.4和5.3μM时抑制Mtb。在测试的革兰氏阳性和革兰氏阴性细菌中,新衍生物对枯草芽孢杆菌具有活性,化合物18 [3-(三氟甲基)苯甲酰基]和29(N-酰基吗啉基)分别显示抑制浓度41和90.7μM,而它们对其他测试细菌菌株没有
-
One-Pot Preparation of Arylethynyl Sulfides and Bis(arylethynyl) Sulfides作者:Qiong Su、Zi-Jian Zhao、Feng Xu、Peng-Cai Lou、Kai Zhang、De-Xun Xie、Lei Shi、Qing-Yun Cai、Zhi-Hong Peng、De-Lie AnDOI:10.1002/ejoc.201201422日期:2013.3An efficient preparation for arylethynyl sulfides 1 and bis(arylethynyl) sulfides 2 has been accomplished through a one-pot, three-step strategy that starts from arylethanonyl sulfides and bis(arylethanonyl) sulfides, respectively, without the use of various terminal arylacetylenes as substrates. When lithium hexamethyldisilazane (LHMDS), ClP(O)(OEt)2, and LHMDS were sequentially added to a tetrahydrofuran
-
[EN] GLUCAGON RECEPTOR ANTAGONISTS, COMPOSITIONS, AND METHODS FOR THEIR USE<br/>[FR] ANTAGONISTES DU RÉCEPTEUR DE GLUCAGON, COMPOSTIONS ET PROCÉDÉ D'UTILISATION DE CES COMPOSÉS申请人:SCHERING CORP公开号:WO2009140342A1公开(公告)日:2009-11-19The present invention relates to compounds of general formula (I), wherein ring A, ring B, R1, R2, R3, Z, and L1 are selected independently of each other and are as defined herein, to compositions comprising the compounds, and methods of using the compounds as glucagon receptor antagonists and for the treatment or prevention of type 2 diabetes and conditions related thereto.本发明涉及一般式(I)的化合物,其中环A、环B、R1、R2、R3、Z和L1彼此独立选择,并如本文所定义,涉及包含该化合物的组合物,以及将该化合物用作胰高血糖素受体拮抗剂并用于治疗或预防2型糖尿病及相关疾病的方法。
表征谱图
-
氢谱1HNMR
-
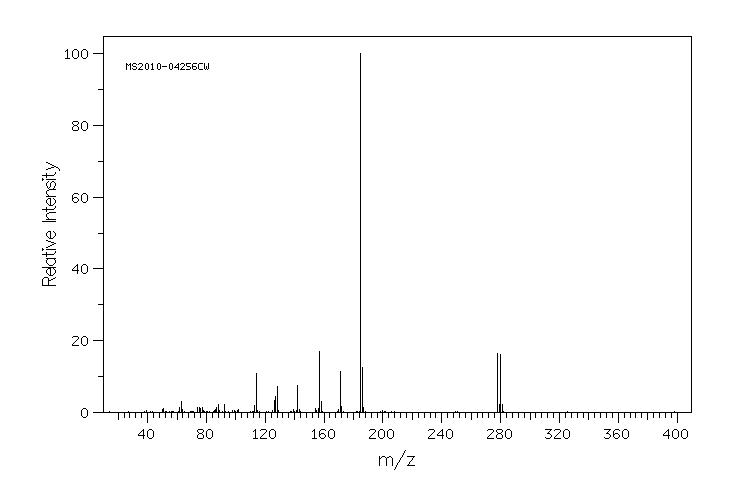
质谱MS
-
碳谱13CNMR
-
红外IR
-
拉曼Raman
-
峰位数据
-
峰位匹配
-
表征信息
同类化合物
(S)-溴烯醇内酯
(R)-3,3''-双([[1,1''-联苯]-4-基)-[1,1''-联萘]-2,2''-二醇
(3S,3aR)-2-(3-氯-4-氰基苯基)-3-环戊基-3,3a,4,5-四氢-2H-苯并[g]吲唑-7-羧酸
(3R,3’’R,4S,4’’S,11bS,11’’bS)-(+)-4,4’’-二叔丁基-4,4’’,5,5’’-四氢-3,3’’-联-3H-二萘酚[2,1-c:1’’,2’’-e]膦(S)-BINAPINE
(11bS)-2,6-双(3,5-二甲基苯基)-4-羟基-4-氧化物-萘并[2,1-d:1'',2''-f][1,3,2]二氧磷
(11bS)-2,6-双(3,5-二氯苯基)-4羟基-4-氧-二萘并[2,1-d:1'',2''-f][1,3,2]二氧磷杂七环
(11bR)-2,6-双[3,5-双(1,1-二甲基乙基)苯基]-4-羟基-4-氧化物-二萘并[2,1-d:1'',2''-f][1,3,2]二氧杂磷平
黄胺酸
马兜铃对酮
马休黄钠盐一水合物
马休黄
食品黄6号
食品红40铝盐色淀
飞龙掌血香豆醌
颜料黄101
颜料红70
颜料红63
颜料红53:3
颜料红5
颜料红48单钠盐
颜料红48:2
颜料红4
颜料红261
颜料红258
颜料红220
颜料红22
颜料红214
颜料红2
颜料红19
颜料红185
颜料红184
颜料红170
颜料红148
颜料红147
颜料红146
颜料红119
颜料红114
颜料红 9
颜料红 21
颜料橙7
颜料橙46
颜料橙38
颜料橙3
颜料橙22
颜料橙2
颜料橙17
颜料橙 5
颜料棕1
顺式-阿托伐醌-d5
雄甾烷-3,17-二酮